Question
Question: What is the hybridization in the central atom of \({{H}_{3}}O+\)?...
What is the hybridization in the central atom of H3O+?
Solution
The central atom in H3O+is Oxygen. It loses one electron to gain a positive charge. H3O+is the cation as it has one positive charge on it. The hybridization of a molecule depends on the number of lone pairs and bond pairs present on the atom.
Complete answer:
To understand it easily, first, let us see the structure of H3O+ ion.
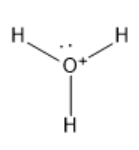
We know that Oxygen is the central atom in the hydronium ion. It has three hydrogen atoms attached to it via a single bond.
As per the electronic configuration of Oxygen, it has 6 electrons in the last shell, but it loses one electron and gains a positive charge. Thus, there are 5 electrons left in the last shell which forms a lone pair of electrons.
To find the hybridization of H3O+ we need to calculate the number of electrons present in the ion.
The formula is: Hybridization (No. of electrons) = 21[V+M−C+A]
We can directly identify the hybridization in a molecule by just knowing the number of electrons.
| No of electrons | Hybridization |
|---|---|
| 2 | sp |
| 3 | sp2 |
| 4 | sp3 |
| 5 | sp3d |
| 6 | sp3d2 |
Now let’s calculate the number of electrons:
No. of electrons = 21[V+M−C+A]
Here,
V= No. of valence electrons in central atom = 6
M = No. of monovalent atoms attached to central atom = 3
C = Charge on cation = 1
A = Charge on Anion = 0
No. of electrons = 21[6+3−1+0]= 21[9−1]= 21[8]=4
The number of electrons is 4, so the hybridization will be sp3hybridization. With an ion having 4 electrons the electronic geometry comes to be tetrahedral.
Note:
To understand it in an easier way, draw the Lewis dot structure of hydronium ions. The structure shows four pairs of electrons that are present in the atom. Out of these three pairs are shared with hydrogen atoms to form bonds and the fourth electron pair is the lone pair.
