Question
Chemistry Question on Chemical bonding and molecular structure
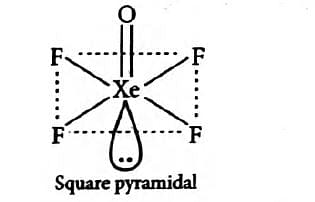
What is the hybridization and geometry of the compound XeOF4 ?
A
sp3d2 and octahedral
B
sp3d and square pyramidal
C
sp3d and trigonal bipyramidal
D
sp3d2 and square pyramidal
Answer
sp3d2 and square pyramidal
Explanation
Solution
Number of hybrid orbitals (X)
=21(V.E.+M.A.−c+a)
For, XeOF4
V.E.=8;M.A. (monovalent atoms) =4
(X)=21(8+4)⇒6
i.e., sp3d2 hybridisation