Question
Question: What is the hybridisation of the oxygen atom in an alcohol \[(\,R - O - H)\] molecule? \[A.\, \,s{...
What is the hybridisation of the oxygen atom in an alcohol (R−O−H) molecule?
A.sp3
B.sp
C.sp2
D.sp3d
Solution
The electronic configuration of oxygen, O=1s2,2s2,2p4 . The oxygen is bound with hydrogen and alkyl groups. The hybridization concept is used in organic compounds to explain chemical bonding. In alcohol, there will be 2σ+2 lone pairs.
Complete step-by-step answer: The hybridization concept is used in organic chemistry to explain chemical bonding. This theory is very useful to explain the covalent bonds in organic compounds.
The hybridization is an intermixing of atomic orbitals of different shapes and has nearly the same energy to give the same number of hybrid orbitals of the same shape, equal energy and orientation such that there will be less repulsion between the hybrid orbitals. $$$$
The electronic configuration of oxygen, O=1S2,2S2,2Px2,2Py1,2Pz1 .
In the ground-state of the oxygen, it has one lone pair and two single electrons which can be shared. But actually, the oxygen has two lone pairs and two single electrons. Due to this condition, the hybridisation will be as follows,
Hybridization of (R−O−H) will be,
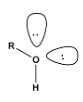
2σ+2 lone pairs
Hybridisation=2+2
So, the hybridisation of oxygen atom is sp3 .
According to the hybridisation, the geometry around the oxygen atom should have tetrahedral and the angle should be ∼109∘28′ . But in reality, it is not observed. The reason is due to the lone pairs of oxygen. They cause repulsion to each other and the resulting bond angle is slightly less than the real value.
Hence, the (R−O−H) is sp3 hybridised and the bond angle is slightly less than the tetrahedral angle.
Note: The hybridisation of oxygen atom is sp3 which means it has four sp3 hybrid orbitals. One of the sp3 orbital overlaps with s orbital from Hydrogen to form O−H σ -bond and one of the sp3 hybridised orbital overlaps with sp3 hybridised orbital of carbon to form C−O σ -bond. The two lone pairs of oxygen are the remaining sp3 hybridised orbital.
