Question
Question: What is the hybridisation of \( {P_4} \) molecules?...
What is the hybridisation of P4 molecules?
Solution
Hint : To know about the hybridisation of the molecule, we have to look for the number of atoms it is connected to, plus the number of lone pairs that particular atom is bearing on itself. The hybridisation also gives us an idea about the shape of the particular molecule.
Complete Step By Step Answer:
Let’s first understand what hybridisation is. So hybridisation is nothing but it is an intermixing of orbitals with the same energy level.
Generally, in a molecule, each atom has its own different hybridisation. But in case of tetra phosphorus P4 , all the atoms have the same hybridisation state.
So, let’s first understand its structure, as phosphorus P , belongs to group V of the periodic table, so it is often assumed that it will make three bonds with other molecules to complete its valence, but due its large size and partially filled p orbitals with three bonds makes its geometry unstable. So to compensate this, phosphorus makes four bonds and forms a tetra atomic molecule.
Let’s look the structure of tetra phosphorus P4
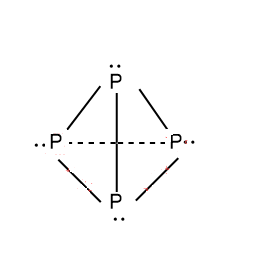
Hence, it is very clear from the structure that the hybridisation state of P in tetra phosphorus P4 is sp3 . As it has 3 bonds plus 1 lone pair of electrons.
So, Hybridisation
= 3 bonds + 1 lone pair of electrons = 4 Thus , sp3 Hybridisation
And the shape is Tetragonal.
Note :
Tetra phosphorus P4 , can only make sigma bonds, and is unable to undergo π (pie bond) formation because of the large atomic size of Phosphorus , P . Though this structure with sigma bonds is also stable.
