Question
Question: What is the hybridisation of \( {C_2}{H_4} \) . Draw its structure also....
What is the hybridisation of C2H4 . Draw its structure also.
Solution
The common name of alkenes is alkylene with the general formula CnH2n , containing double bonds between the carbon atoms. Alkenes are also called unsaturated hydrocarbons. Alkenes are usually sp2 hybridized with a planar geometry. Ethylene or ethene is the simplest alkene.
Complete answer:
C2H4 has sp2 hybridization.
C2H4 has 2 carbon molecule and 4 hydrogen molecule, each carbon atom is attached to other carbon by double bond and singly bonded to the hydrogen atom.
Ground state electronic configuration of carbon is 1s2 2s2 2p2 . Because of the approach of hydrogen atom carbon acquires the electronic configuration 1s2 2s1 2px1 2py1 2pz1 but only 1s and 2p orbital will take part in hybridization. Ethene has 3sp2 hybridized; this results in formation of orbitals.
One electron from 2s2 orbital moves to 2pz orbital.
sp2 hybrid orbital of one carbon overlaps with sp2 hybrid orbital of second carbon atom and results in sp2−sp2 overlap (sigma overlap) and the two sp2 hybrid orbitals is head on overlapped by two hydrogen atom which has unpaired electrons. So now total 5 sigma bonds.
There is still one p -orbital that has an unpaired electron, in each of the carbon atom there is one p -orbital that is unhybridized so now there will be overlap between the 2 lobes of unhybridized p -orbital and hence one pi bond will be formed.
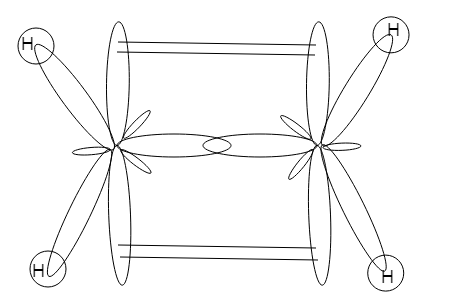
Around each of the carbon atoms the bond angle is near to 120∘ . 117.4∘ is the H−C−H angle and the shape thus formed is trigonal planar.
Note:
Ethylene is a growth hormone that affects the ripening and flowering in plants. Ethylene contains one pi bond and five sigma bonds. Ethylene mostly undergoes electrophilic substitution reactions. Ethylene is used in industrial reactions such as polymerization, oxidation, and halogenation, etc.
