Question
Question: What is the hybrid state of the central atom in the following \(P{F_5},\,C{O_2},\,B{F_4}^ - \) ....
What is the hybrid state of the central atom in the following PF5,CO2,BF4− .
Solution
Hybridisation is that concept of chemistry in which we study the mixing of atomic orbitals in order to form a new hybrid orbital with variation in energy, shape, from the parent orbital’s. These new orbits are suitable for the pairing of electrons in order to form new bonds so that a molecule can be made.
Complete answer:
In the first step we count the total valence electrons (T.V.E) of the given central metal atom in the molecule.
Then to calculate the numbers of sigma bonds, divide the T.V.E by 8 .
In case after the division of T.V.E by 8 the remainder is left, divide it by 2 for the calculation of lone pairs.
Now calculate the Steric number by adding the lone pair value and the sigma bond value.
In the case of PF5
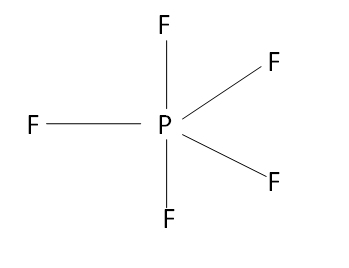
The number of sigma bonds is 5 thus the hybridization of the central metal is sp3d .
In the case of CO2
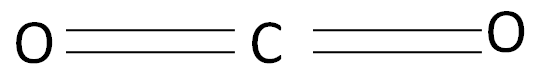
The hybridization of the central metal is sp .
In the case of BF4−
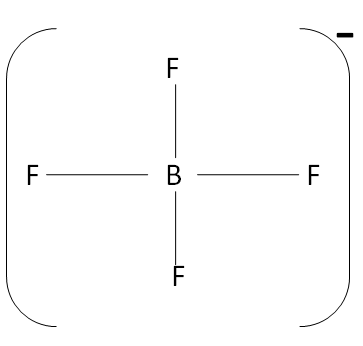
The hybridization of the central metal is sp3 .
Note:
The Steric number calculated for the calculation of the hybridization of the central metal atom in the above case follows the chronology which is as follows: - Iff the Steric number is 2 then the hybridization is sp . Iff the Steric number is 3 then the hybridization is sp2 . Iff the Steric number is 4 then the hybridization is sp3 . Iff the Steric number is 5 then the hybridization is sp3d . Iff the Steric number is 6 then the hybridization is sp3d2 . . Iff the Steric number is 7 then the hybridization is sp3d3 .
