Question
Question: What is the ground state electron configuration of a \({}_{27}Co\) atom in the gas phase?...
What is the ground state electron configuration of a 27Co atom in the gas phase?
Solution
The elements in the periodic table are arranged in order of increasing atomic number. Atomic number is the total number of electrons present in an atom. The electronic configuration of an atom is the filling of electrons in the increasing energy levels or the sub shells (s, p, d, f) according to the Aufbau principle.
Complete answer:
Electronic configuration of any atom tells us the total number of electrons in that atom which is equal to the atomic number of that element and also the total number of protons. Electronic configuration of any element consists of filling the orbital or subshells (s, p, d, f) with electrons. The filling of electrons in various orbitals is according to a principle of Aufbau that takes place from the lower energy level to the higher energy level. The s, p, d, f subshells are written along with the number of their shell, like 1, 2, 3, etc.
The ground state of any atom is the state in which it is neutral and is the gas phase of cobalt, meaning there is no transfer of electrons to a higher or lower energy level. Cobalt is the element of the d block with atomic number 27. It is neutral when all the atoms are filled according to the Aufbau principle. Therefore, the ground state configuration of cobalt will be, 1s22s22p63s23p64s23d7.
Hence, the ground state electron configuration of a27Coatom in the gas phase is 1s22s22p63s23p64s23d7.
Note:
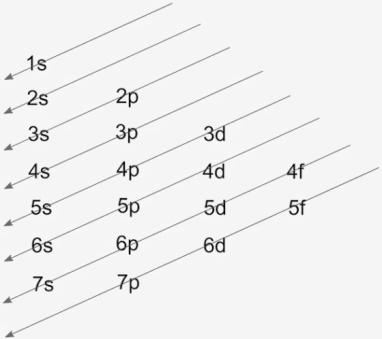
The shorthand electron configuration, which is a noble gas configuration of cobalt is [Ar]4s23d7. The filling of electrons is according to the lower energy levels to the higher ones. This is identified through Aufbau diagram that is,
The ground state is the neutral state, while when an electron gets excited and absorbs energy it moves to the higher energy level and is called an excited state.
