Question
Question: What is the geometry of oxygen in \( C{H_3}OC{H_3} \) ....
What is the geometry of oxygen in CH3OCH3 .
Solution
Hint : In this we want to know about the geometry of the bond or we can say molecular shape of the bond. This dimethyl ether has oxygen which is covalently bonded with two carbon atoms. To know the exact geometry of this, we need to know about the lone pairs present in it.
Complete Step By Step Answer:
To know about the geometry of CH3OCH3 , we know that oxygen will be the central atom. As there are two C−O bonds in this structure as well as on oxygen we will have lone pairs of electrons also. When we consider stability the most stable geometry of electron pairs, bonding non-bonding is tetrahedral. Oxygen in the central close to lone pair tries to decrease the angle C−O−C which makes the bond angle equal to 105o then 109.5o .
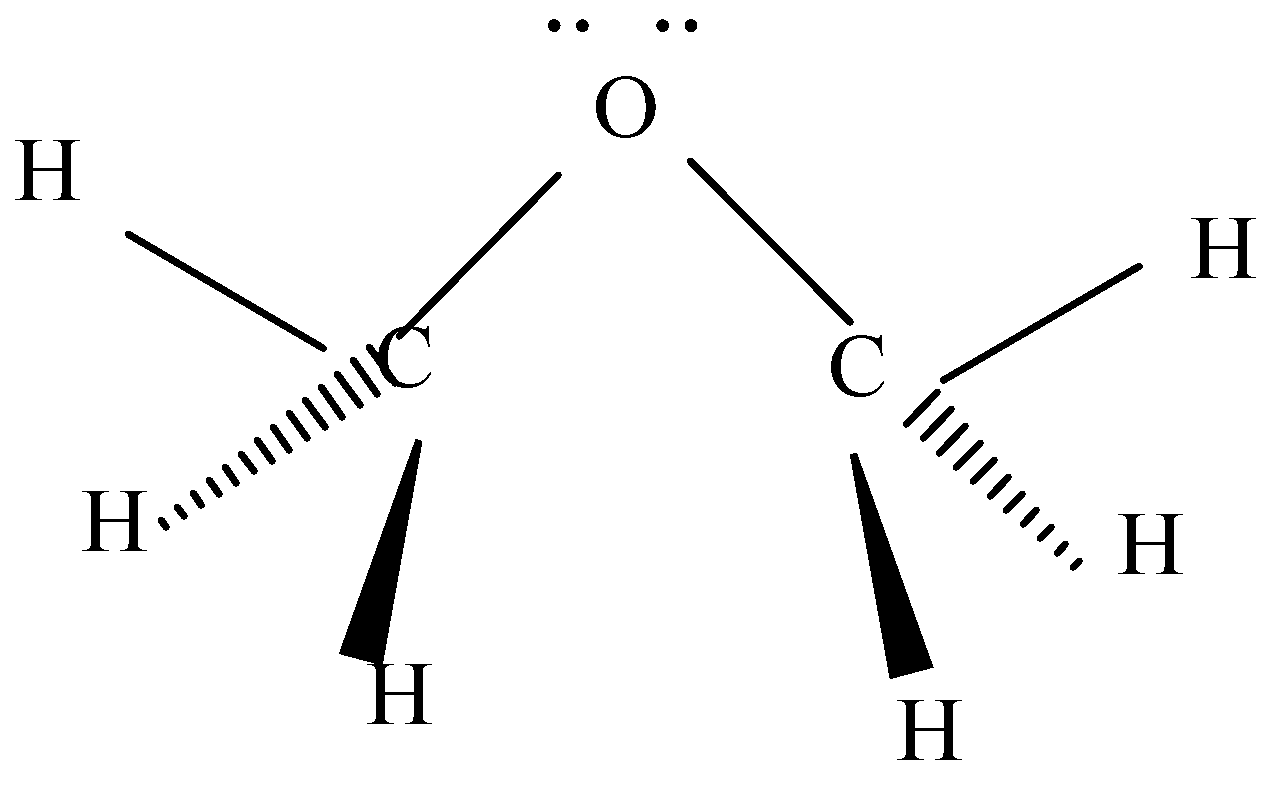
Note :
Oxygen is hybridized as sp3 and it has a dipole moment of 1.3D . Generally, to determine geometry of any structure we use Lewis structure or VSEPR theory. There are some particular steps to follow to describe the geometry of any structure while using VSEPR theory.
