Question
Question: What is the geometry and hybridization of \[AuC{l_4}^ - \]?...
What is the geometry and hybridization of AuCl4−?
Solution
Gold is one of the transition elements in the periodic table. The atomic number of gold is 79and the mass number of gold is 196.967. The symbol of gold is Au. In chemistry, the periodic table plays a vital role. In the periodic table there are a total of 118 elements. In the periodic table there are a total of 18 columns and 7 rows. The columns are called groups. Hence, 18 groups in the periodic table. The rows are called the period. Hence, a total of 7 periods are on the table.
Complete answer:
The geometry of AuCl4− is square planar. Because of the weak ligand nature of the chlorine atom.
The geometry of AuCl4− is square planar is given below,
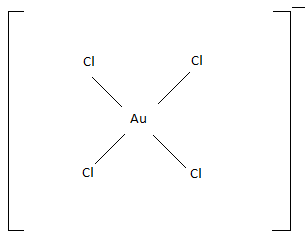
The hybridization of AuCl4− is dsp2 due to the atomic nature of the gold and d orbitals.
According to the above discussion, we conclude the geometry and hybridization of AuCl4− is square planar and dsp2.
Note:
In the periodic table, a total of 118 elements are present now. In the periodic table there are eighteen groups and seven periods. The Halogen group is the seventeenth group in the periodic table. In this group, four elements only consider halogen out of five elements in the column. There are fluorine, chlorine, bromine, and iodine considered halogen in the modern periodic table. Halogen is the most electron affinity group in the periodic table. Halogen is also the most electronegativity group in the periodic table. The ionization energy of the halogen is very less compared to other groups. In halogen from top to bottom, electron affinity decreases. Chlorine is the highest electron affinity species in the periodic table, because the size of the fluorine is very small compared to chlorine. But, the electronegativity value of fluorine is high compared to the electronegativity value of chlorine.
