Question
Question: What is the functional group of alcohol and phenol? A) \[ - OH\] Phenol and  −OH Phenol and 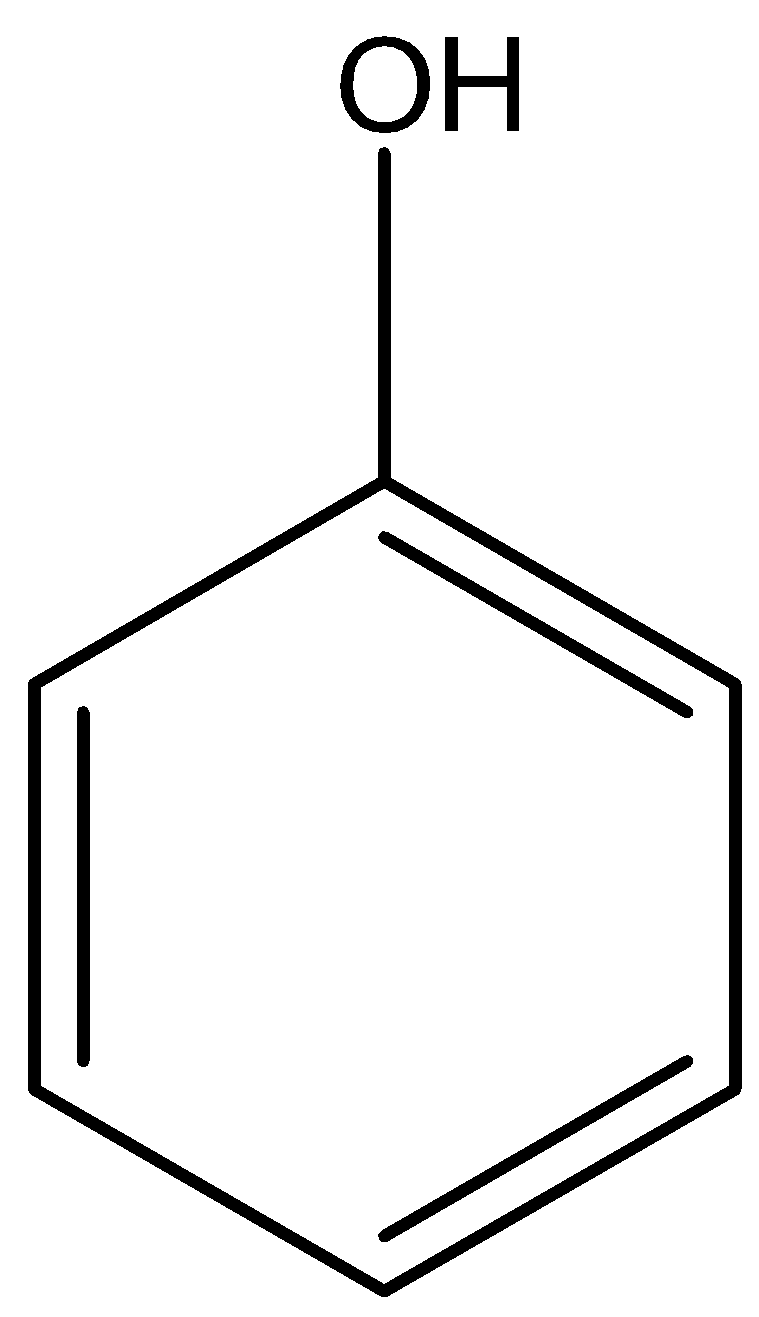 alcohol
alcohol
B) 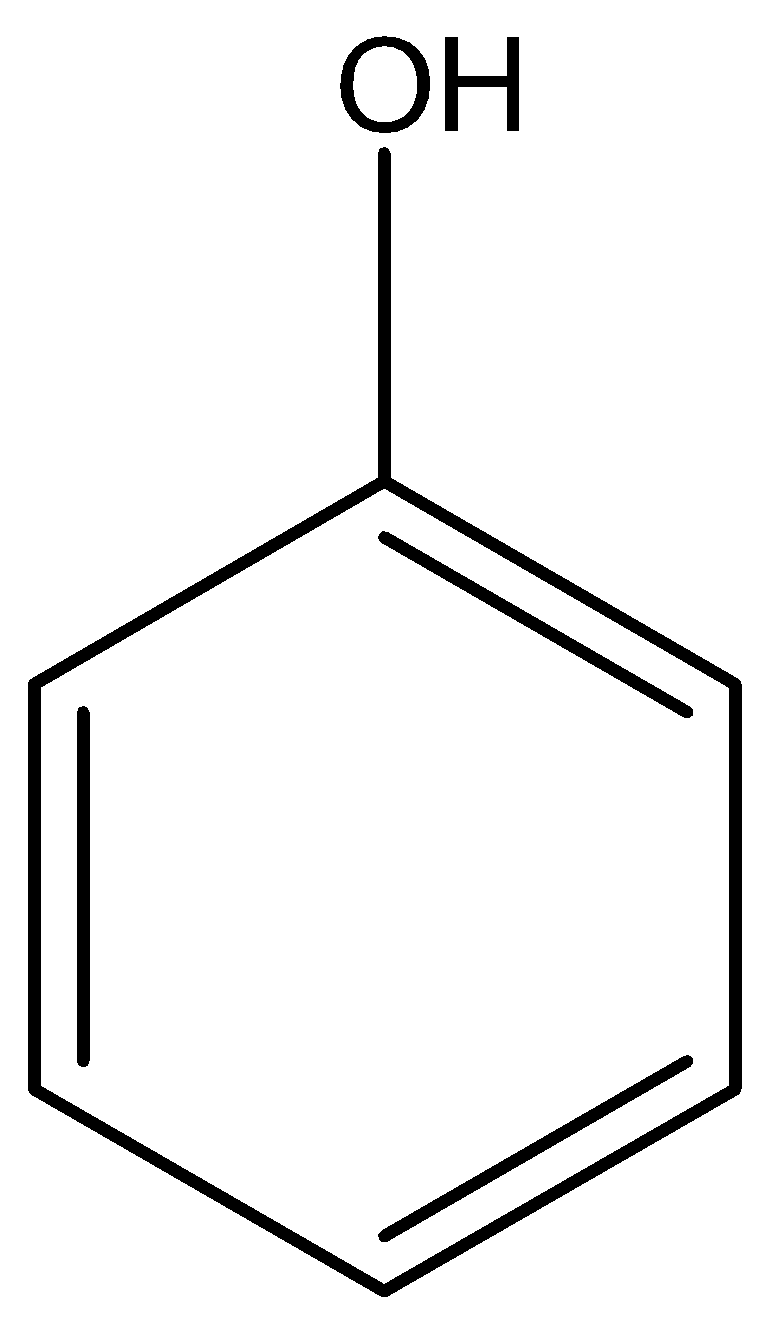 Phenol and −OH alcohol
Phenol and −OH alcohol
C) −OH Alcohol and −COOH Phenol
D) None of the above
Solution
The general formula of alcohol is CnH2n+1OH. Alcohol and phenol is the two organic compounds containing the hydroxyl group i.e. −OH in common.
We have to know that alcohol is an organic compound which has at least one hydroxyl group which is attached to the saturated carbon atom chain.
Complete step by step answer:
We have to remember that the nomenclature for writing the name of alcohol is Suffix “ol” is used when hydroxyl group is present as a higher priority in any compound whereas Prefix “hydroxy” is used when other groups are present as higher priority than alcohol.
Examples of alcohol:
C2H5OH- Ethanol
Phenol is an aromatic organic compound that contains hydroxyl group which is attached to a benzene ring therefore the functional group is −C6H5OHor 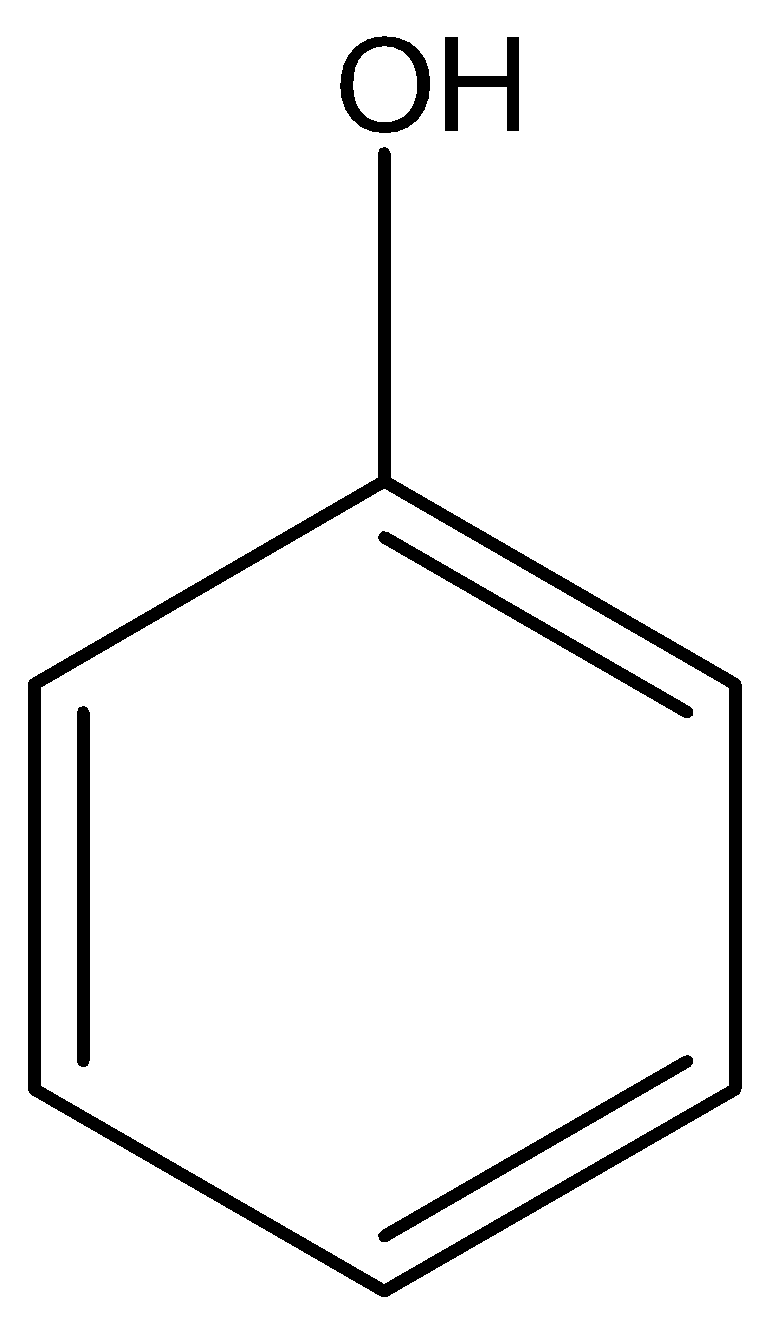
Image created in chemdraw by SME.
Phenol is a weak acid that has pH of 8−12 , there is resonance stabilization in phenol due to which it is more acidic than aliphatic alcohols.
Option A) this is an incorrect option as −OH is a functional group of alcohol and 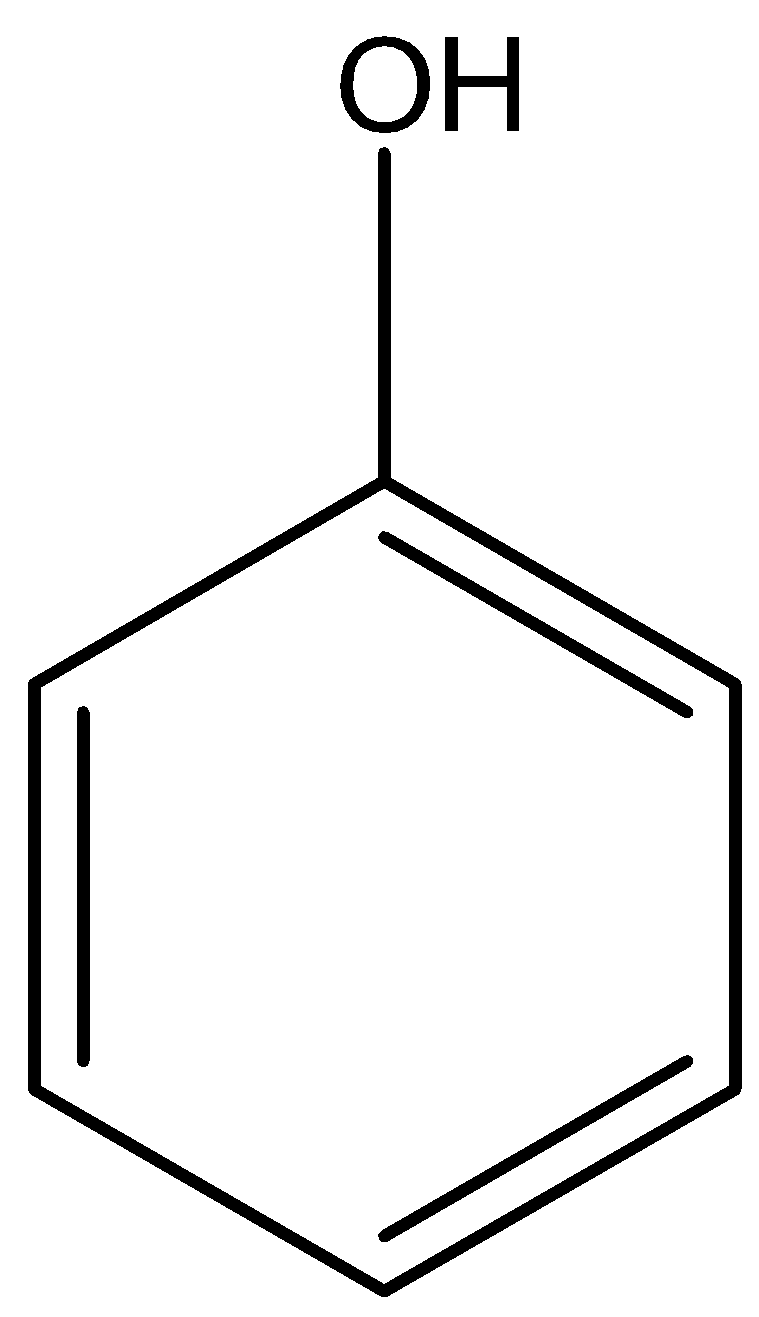 is the functional group of phenol.
is the functional group of phenol.
Option B) This is a correct option as the two represent phenol and alcohol in the correct way.
Option C) this is an incorrect option as −OH is a functional group of alcohol but −COOH is a functional group of carboxylic acid and not phenol.
Option D) this is an incorrect option as we get option B as the correct option.
Hence, the correct option is, ‘Option B’.
Note: We need to know that an alcohol is an aliphatic organic compound whereas Phenol is an aromatic organic compound. Phenols have generally higher acidity than alcohols.
