Question
Question: What is the formula of allyl isocyanide? How many sigma and pi bond are present in it?...
What is the formula of allyl isocyanide? How many sigma and pi bond are present in it?
Solution
Isocyanide is an organic compound with the functional group −N≡C . It Is an isomer of the related nitrile. Hence the prefix is isocyano. An allyl group is the substituent with the structural formula H2C=CH−CH2R , where R is the rest of the molecule.
Complete answer:
The formula of allyl isocyanide is C4H5N .
Sigma bonds (σ) are the strongest type of covalent bond, formed by head-on overlapping of atomic orbitals.
Pi bonds (π) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals.
Every single bond consists of one sigma bond, and that double bond is made up of one sigma and one pi bond. A triple bond consists of one sigma and two pi bonds.
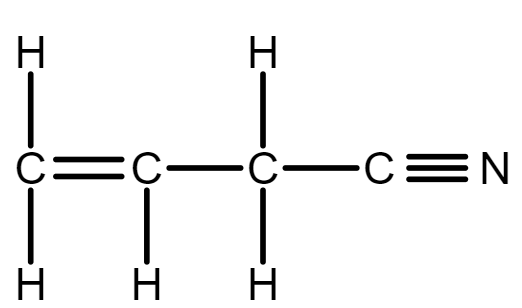
In allyl isocyanide, there is a 5 single bond between carbon and hydrogen C−H , and we know in double bond there is one sigma and one pi bond therefore, Three sigma bond between carbon and carbon atoms C−C . And a triple bond contains one sigma bond and two pi bonds, so there is one sigma bond between carbon and nitrogen atom C−N .
Total sigma bonds are: 5(C−H)+3(C−C)+1(C−N)=9σ bonds.
One pi bond between (C−C) and two pi bonds between (C−N) , therefore, total pi bonds are: 1(C−C)+2(C−N)=3π bonds.
So, there are 9σ bonds and 3π bonds in allyl isocyanide.
Note:
There are two non bonded electron pairs in allyl isocyanide, these non bonded electrons are of nitrogen as it has 5 valence electrons in its outermost shell. It requires 3 electrons to fulfill its outermost shell, thus Nitrogen makes a triple bond with carbon to get stable.
