Question
Question: What is the formula for the compound that forms when magnesium bonds with phosphorus?...
What is the formula for the compound that forms when magnesium bonds with phosphorus?
Solution
Hint : First we will determine the valency of magnesium and phosphorus. We will use the ions of magnesium and phosphorus to get the valency of both the elements. Once valency is determined, we will simply use the crossover rule to form the compound and hence name it accordingly.
Complete Step By Step Answer:
When magnesium (Mg) bonds with phosphorus (P) , it will produce magnesium phosphide (Mg3P2) .
Magnesium has an atomic number of 12 which means that to attain a stable octet configuration, it will tend to lose 2 electrons and hence it will become a positive ion with +2 charge. Similarly, phosphorus has an atomic number of 15 . Therefore, to attain a stable octet configuration, phosphorus will tend to gain 3 more electrons. Hence, it will become a negative ion with −3 charge.
We know that the valency of an ion is the same as the charge on that ion. Hence, the valency of Mg+2 is 2 and the valency of P3− is 3 .
Now, to form a compound of magnesium and phosphorous, we will use the crossover rule to cross the valency of each element to one another as shown:
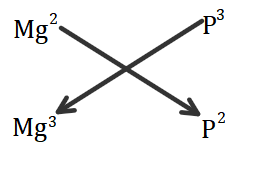
Hence the valency gets swapped and the compound formed is Mg3P2 , magnesium phosphide.
Magnesium phosphide is an ionic compound since it formed by the exchange of electrons.
Note :
In stable octet configuration, all the orbits of an element are completely filled with electrons. For example, after losing two electrons the two remaining shells are completely filled with electrons (having 2 in the first shell and 8 in the second shell). Be careful while determining the charge of magnesium and phosphorus ion. If you do not know the atomic number of the elements then just use a periodic table.
