Question
Question: What is the formula for the compound iodous acid \(\left( HI{{O}_{3}} \right)\)?...
What is the formula for the compound iodous acid (HIO3)?
Solution
The compound as described above i.e. iodous acid is the oxoacid of iodine (iodine oxoacid). In chemistry, iodous acid is commonly known as hypo-iodic acid (conjugate acid of an iodide and an iodine oxoacid).
Complete answer:
Let us study the compound in detail
Iodous acid –
It has the molecular weight of 159.911g/mol having molecular formula as HIO2. The structure of the same is as described below-
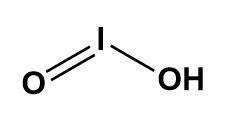
Here, we can see that the number of hydrogen bond donors is one whereas, the number of hydrogen bond acceptors are two.
To note, there are three (worthy) types of acids in the association with iodine;
Iodic acid i.e. HIO3 .
Iodous acid i.e. HIO2 .
Hypo-iodous acid i.e. HIO .
Note:
The oxides of iodine (depending upon the oxidation state of iodine) formed in trace quantities are listed below;
Di-iodine monoxide i.e. I2O .
Iodine monoxide i.e. IO .
Iodine dioxide i.e. IO2 .
Iodine tetroxide i.e. (IO2)2 .
Iodine pentoxide i.e. (O(OI2)2) .
