Question
Question: What is the formal charge on the Br and O atoms in the \(BrO_3^ - \) ion?...
What is the formal charge on the Br and O atoms in the BrO3− ion?
Solution
The BrO3− Lewis structure has a total of 26 valence electrons. This includes the electron represented by the negative charge in BrO3− ion. You need to put brackets around the BrO3− Lewis structure as well as a negative charge to show that the structure is a negative ion. To get the most stable structure, there are double bonds and one single bond attached to Br, along with a lone pair.
Complete answer:
BrO3− ion has a total of 26 valence electrons. In the Lewis structure, Br is placed in the center since it is lower in electronegativity. With all single bonds connecting the atoms, the formal charge of the O atoms are each −1 while the Br is 2. However, it is best to reduce the formal charges as much as possible since doing so would yield a more stable structure. With double bonds, Br and two O atoms would have a formal charge of 0, thus leaving the remaining O atom with a charge of −1.
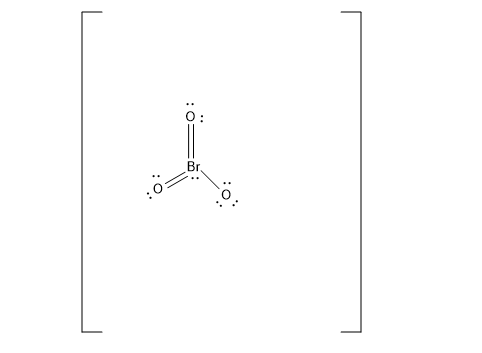
Note:
The number of electrons around the atom of interest determines the formal charge of that atom; single bonds share the electrons between the bound atoms, lone pairs devolve solely to the atom concerned. Of course in the actual molecule, all of the oxygen atoms are equivalent. The formal charge on the oxygen is 0. The bromine has a formal charge of 0, and this is still −1. The total formal charge here is −1.
