Question
Question: What is the formal charge on each atom in \( {{C}_{2}}{{H}_{3}}Cl \) ?...
What is the formal charge on each atom in C2H3Cl ?
Solution
We know that the formal charge of an atom says about the charge that is assigned to it in a molecule and it can be calculated if you know about the number of valence electrons, number of nonbonding electrons or lone pair of electrons and the total number of sigma bonds which is shared in covalent bond of that atom in its ground state.
Complete answer:
The charge assigned to an atom in a molecule is known as formal charge.
Formal charge is the charge we would assign to an atom in a molecule if we assume that the electrons in the bonds the atom makes are shared equally between itself and the other atom, regardless of the two atoms' electronegativity. In order to determine the formal charges on all the atoms in C2H3Cl , or vinyl chloride, draw its Lewis structure.
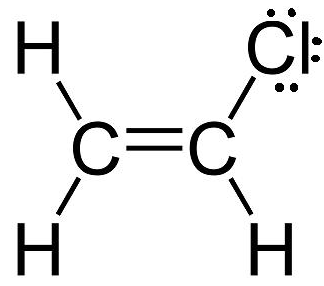
The vinyl chloride molecule has 18 valence electrons − 4 from each C atom, 1 from each H atom, and 7 from Cl − all of which are accounted for by the above Lewis structure. The easiest way to determine formal charge for an atom is to compare its valence electrons with the number of electrons it "gets" in a molecule. Let's start with the carbon atoms, since they both form 4 bonds. The assumption made for determining formal charge is that the electrons in a bond are shared equally. This means that 4 bonds will give this carbon atom 4 electrons.
Now compare this number to the atom's valence electrons. Since carbon has 4 valence electrons, and it gets 4 electrons, its formal charge will be zero.
The same reasoning applies to each of the three hydrogen atoms as well. Each hydrogen atom forms a 1 bond, so it gets 1 electrons from the two that form the bond. Since hydrogen has a 1 valence electron, its formal charge will be zero. Now for the chlorine atom. Chlorine has 7 valence electrons. As you can see in the Lewis structure, it has 6 electrons as lone pairs and gets 1 more electrons from the bond it forms with carbon.
Note:
Remember that The valence electrons are the electrons which are present in the outermost shell of an atom and these can take part in chemical bonding. The non-bonding electrons are those electrons which do not take part in the chemical bonding and they are also referred to as lone pairs of electrons.
