Question
Question: What is the formal charge of \({ NO }_{ 2 }^{ - }\)?...
What is the formal charge of NO2−?
Solution
Hint: The formal charge is the difference in the number of valence electrons in the atom and the number of valence electrons in the Lewis structure.
Complete answer step-by-step:
The formal charge of an atom in a molecule can be calculated by the following equation;
FC=V−N−B÷2
where V= number of valence electrons of the atom in the free atom
N = number of nonbonding electrons on this atom in the molecule
B = Total number of electrons shared in covalent bonds with other atoms in the molecule.
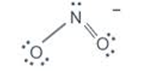
Nitrogen in the nitro group NO2− : FC= 5−2−6÷2=0
Double bonded oxygen in NO2− : FC=6−4−4÷2=0
Single bonded oxygen in NO2− : FC= 6−6−2÷2=−1
Hence, 0+0+(−1)=−1 as expected for NO2−
Additional Information:
Formal charge means ignore the lone pair of electrons and electrons shared by that atom from valence electrons then whatever is left with you is the significance of formal charge.
- It helps us to keep track of the electrons in a molecule.
- It tells you whether an atom has more electrons or protons associated with it.
- It allows us to assign electrons to a particular atom in a molecule.
Note: The possibility to make a mistake is that you may confuse between formal charge and the actual charge. But keep in mind that formal charges do not represent the actual charge on atoms in a molecule. The formal charges are assigned to the atoms in the Lewis structure to keep track of the electrons involved in the bonding.
