Question
Question: What is the empirical formula of the hydrocarbon \[\left( {{C_x}{H_y}} \right)\] that produced the m...
What is the empirical formula of the hydrocarbon (CxHy) that produced the mixture of products in the diagram after a combustion reaction?
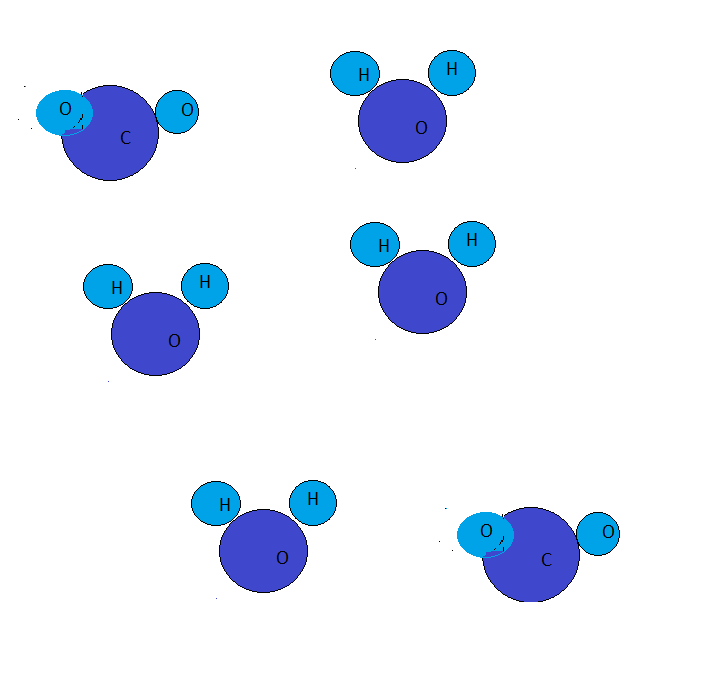
A.CH4
B.C2H8
C.C3H4
D.CH2
Solution
Empirical formula is the shortest representation of molecular formula. All the atoms present in the molecular formula will be in the empirical formula. Alkanes are hydrocarbons when treated with oxygen forms carbon dioxide and water. These reactions are known as combustion reactions.
Complete answer:
Alkanes are saturated hydrocarbons consisting of only carbon and hydrogen atoms. When alkanes are treated with air in presence of oxygen gas leads to the formation of carbon dioxide along with water.
Given that an alkane undergoing combustion reaction produces six moles of water and two moles of carbon dioxide. Thus, the number of carbon atoms in the alkane are two and the number of hydrogen atoms are eight.
This leads to the result that alkane consists of four carbon atoms and eight hydrogen atoms.
Thus, the molecular formula of an alkane is C2H8.
The empirical formula is the shortest representation of a molecular formula. The lowest ratio of atoms of the molecular formula can be written as CH4
The empirical formula of a hydrocarbon (CxHy) that produced the mixture of products in the diagram after a combustion reaction is CH4.
Therefore, Option A is the correct one.
Note:
The combustion reaction is the reaction of alkanes with oxygen. All the atoms in alkane were formed as carbon dioxide and water. By looking at the products the molecular formula can be written. In the given options both molecular formula and empirical formula were there. But the empirical formula is the simplest ratio and it should be considered.
