Question
Question: What is the electrophile in the nitration of benzene reaction ?...
What is the electrophile in the nitration of benzene reaction ?
Solution
Hint : In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid (as occurs in the synthesis of nitroglycerin).Typical nitration syntheses apply so-called "mixed acid", a mixture of concentrated nitric acid and sulfuric acids. This mixture produces the nitronium ion ( NO2+ ), which is the active species in aromatic nitration.
Complete Step By Step Answer:
Benzene is highly prone to electrophilic substitution reactions compared to addition reactions as it loses its aromaticity during addition reaction. As benzene contains delocalized electrons spanning over carbon atoms in the ring, it is highly attractive to electrophiles and is also highly stable to electrophilic substitutions. Generally, the electrophilic substitution reaction of benzene is a three-step process involving: 1)Generation of the electrophile. 2)Intermediate carbocation formation. 3)Removal of a proton from carbocation intermediate Benzene reacts with concentrated nitric acid at 323-333k in the presence of concentrated sulphuric acid to form nitrobenzene. This reaction is known as nitration of benzene. The mechanism for nitration of benzene: Step 1: Nitric acid accepts a proton from sulphuric acid and then dissociates to form nitronium ion. Step 2: The nitronium ion acts as an electrophile in the process which further reacts with benzene to form an arenium ion. Step 3: The arenium ion then loses its proton to Lewis base forming nitrobenzene.
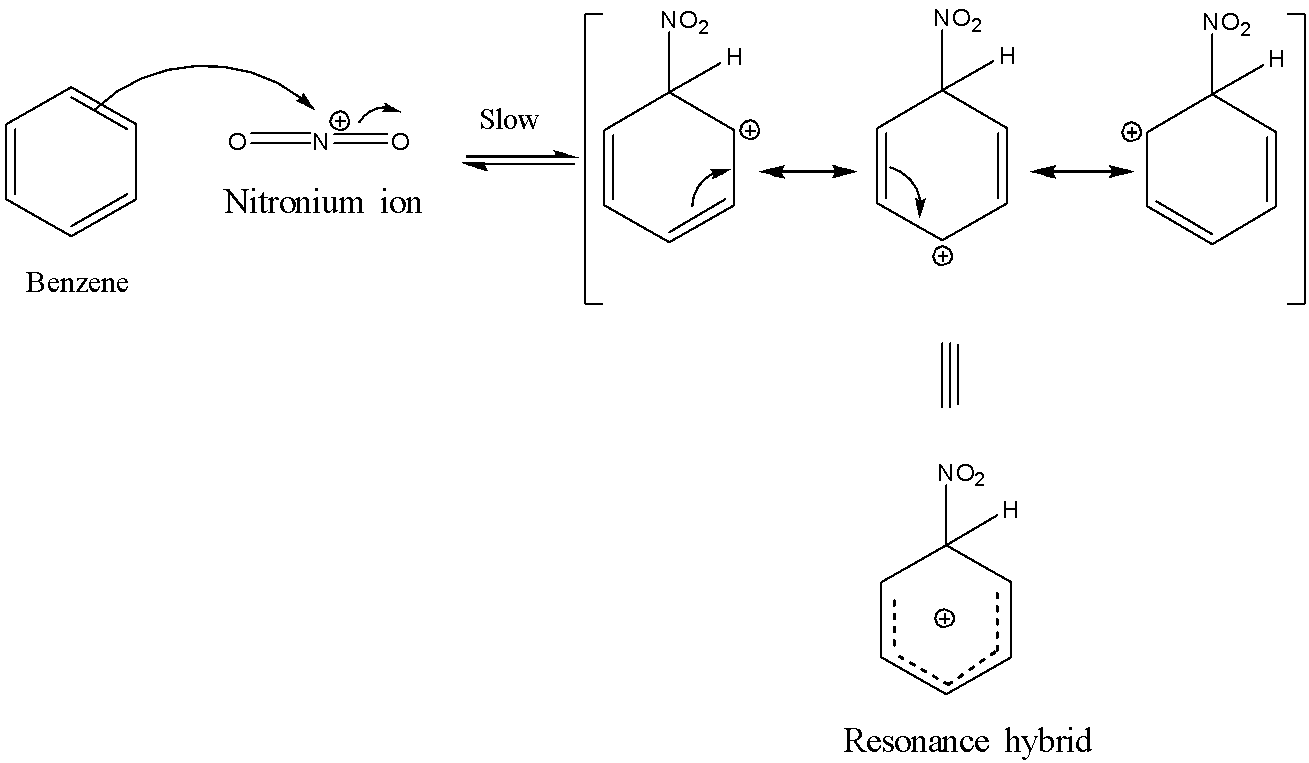
Note :
Not to be confused with Nitrification and nitration . In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid (as occurs in the synthesis of nitroglycerin). Nitrification is the biological oxidation of ammonia to nitrite followed by the oxidation of the nitrite to nitrate occurring through separate organisms or direct ammonia oxidation to nitrate in comammox bacteria.
