Question
Question: What is the electron dot structure of \(NO_{3}^{-}\) ....
What is the electron dot structure of NO3− .
Solution
Lewis dot structure is nothing but the electron dot structure. In Lewis dot structure of the chemical compounds the electrons which are present in the atoms are going to be represented with dots around the respective atom.
Complete answer:
- In the question it is asked what is the electron dot structure of NO3− .
- First of all we should know the normal structure of the given chemical compound.
- The structure of the given molecule NO3− is as follows.
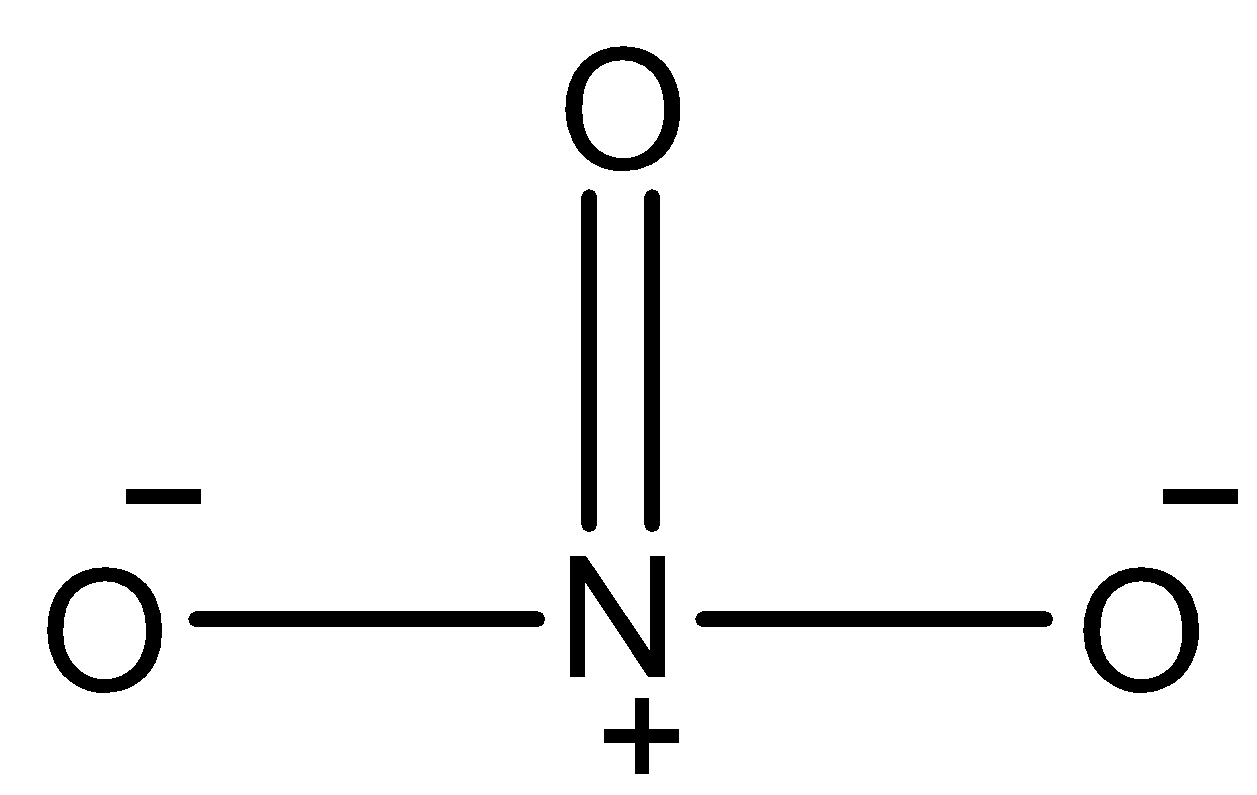
- We have to calculate the number of electrons present in the given molecule.
- The number of electrons which are present in the nitrate anion = 5+ (6) (3) +1 = 24.
- Now we can represent the Lewis dot structure of the nitrate molecule easily and it is as follows.
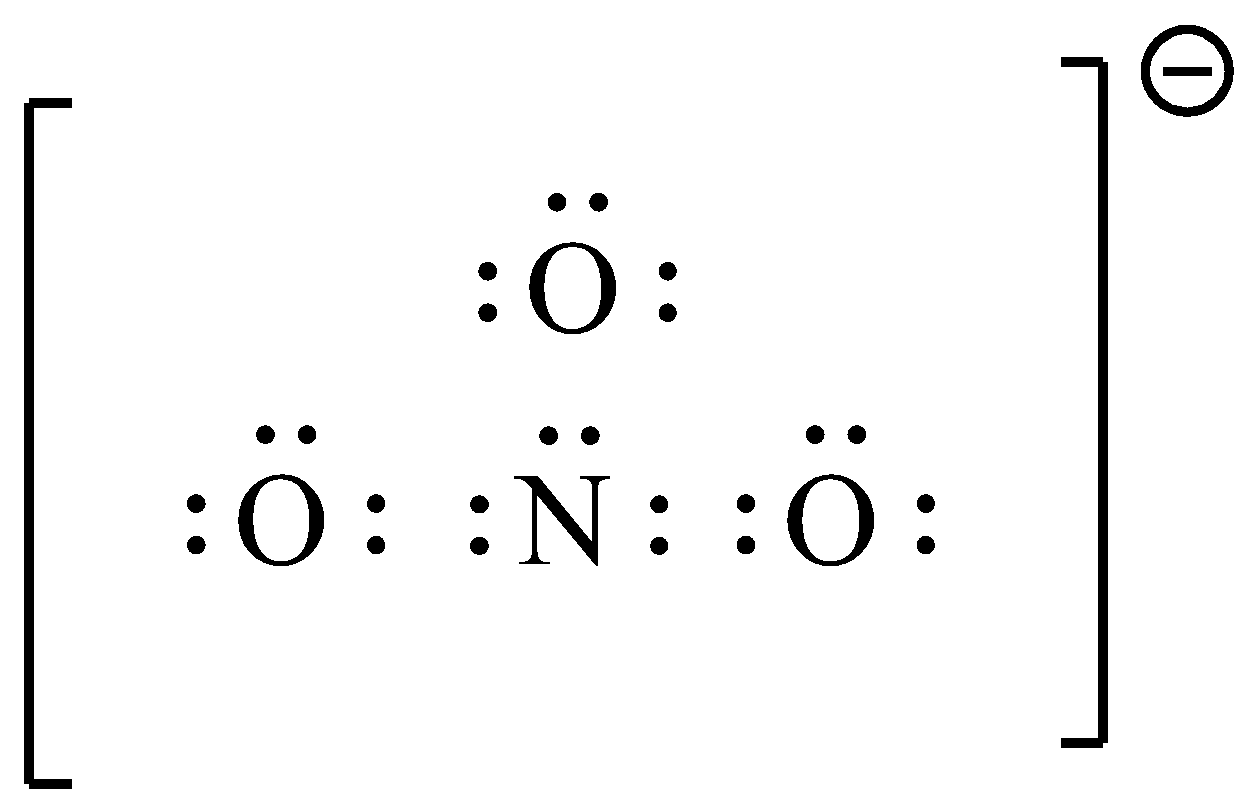
- From the Lewis dot structure we can calculate the number of lone pairs of electrons which are present in the given compound.
- In the given compound each oxygen atom contains two lone pairs of electrons which are not going to be involved in the bonding.
- There is only one lone pair of electrons on the nitrogen atom which is not going to participate in the bonding.
- Therefore the total number of lone pair electrons present in the nitrate anion are seven.
Note:
By using Lewis dot structure or electron dot structure we can find the number of lone pairs of electrons present on the atoms which are present in the structure of the chemical compound.
