Question
Question: What is the electron dot structure of hydrogen chloride?...
What is the electron dot structure of hydrogen chloride?
Solution
We need to know that the hydrogen chloride is also known as hydrochloric acid. It is composed of two atoms hydrogen and chlorine. The simplest covalent bond is formed between two hydrogen atoms. Each hydrogen atom has a single electron, and each needs two electrons for a full outer shell.
Complete answer:
We have to remember that the hydrogen molecule H2 consists of two hydrogen atoms by sharing the two valence electrons. Hydrogen can also form covalent bonds with other atoms as well. For example, hydrogen and chlorine each need one more electron to achieve a noble gas configuration. By sharing valence electrons (each atom donates one), the stable hydrogen chloride i.e. HCl molecule is formed.
Electron dot structure of hydrogen chloride can be represented as:
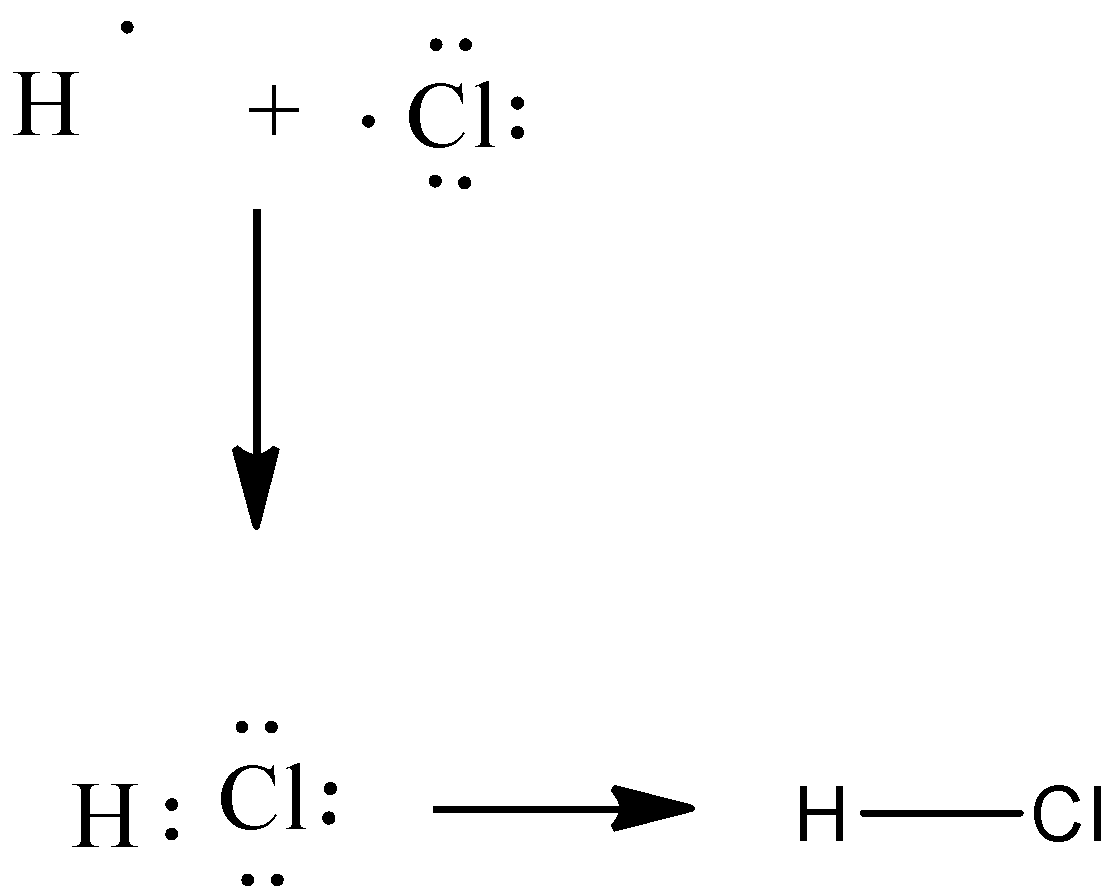
Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. H have 2 valence electrons as a result of sharing with Cl and the Cl has 8 valence electrons as a result of sharing. Hydrogen chloride is an acid which is commonly called hydrochloric acid. The geometry of this molecule is linear and has 180∘.
Note:
We need to remember that the chlorine atom pulls on the bonded pair of electrons harder than the hydrogen atom. This means that the electrons are not shared evenly. Some students may begin to speculate that this would cause a partial charge and that HCl would therefore be attracted to a charged wand.
