Question
Question: What is the electron configuration of \({{F}^{-}}\)?...
What is the electron configuration of F−?
Solution
The configuration which shows how electrons are arranged in an atom, molecule, or an ion in various atomic shells and subshells or molecular orbitals is known as electronic configuration. The atomic number of Fluorine is 9.
Complete answer: In an atom, there are various shells in which electrons orbit around the nucleus of an atom, and these shells consist of various subshells, which further consist of various orbitals. The existence of these shells, subshells, and orbitals was proposed by Rutherford and Bohr.
Now, the filling of electrons in an atom is governed by the Aufbau principle which states that the electrons are filled in the atomic orbitals in increasing order of the energy level of the orbital.
The electrons in an atom are arranged and added in the following sequence according to the diagonal rule.
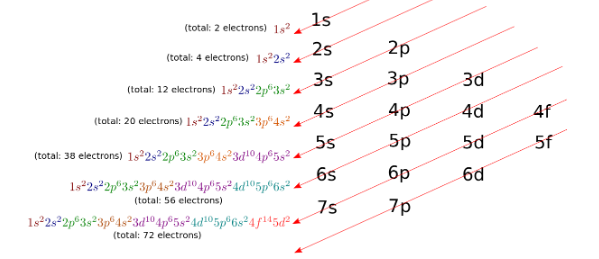
Where the shells are given by the principal quantum number n=1,3,3,4…. The nth shell of an atom can have a maximum 2n2 number of electrons.
The subshells are given by azimuthal quantum number ℓ=0 to (n-1) and hence the subshells s, p, d, and f. The maximum number of electrons in a subshell is given by 2(2ℓ+1).
We know that there are 9 electrons in an F atom and upon the addition of one electron in the outermost shell p, the F− ion is formed.
Since the electronic configuration of F is
F=1s22s22p5
The electronic configuration of F− will be
F−=1s22s22p6
Note: The addition of an electron in the outermost shell of the fluorine atom makes its outermost electron shell L (n=2) complete since the L shell can have a maximum of 8 electrons. This makes the fluorine ion extremely stable and has inert-like features according to the octet rule. Also, the high electron affinity of the fluorine atom is because it can achieve a full outermost electron shell configuration with just the addition of one electron.
