Question
Question: What is the electron and molecular geometry of \[BeC{l_2}\]?...
What is the electron and molecular geometry of BeCl2?
Solution
The VSEPR hypothesis, or Valence Shell Electron Pair Repulsion Theory, and the hybridization of the central atom in the molecule can be used to answer this question. According to the hypothesis, the number of electron pairs on the central atom affects the arrangement of these lone pairs surrounding the central atom in compounds with the chemical formula XYm.
Complete answer:
Beryllium chloride, also known as BeCl2, is an inorganic chemical. At room temperature, it appears as a white or yellow crystal solid. It is available in monomeric and 1-D polymeric forms. Because of the diagonal relationship between beryllium and aluminum, the characteristics of beryllium chloride are comparable to those of aluminum chloride.
Beryllium chloride has a molar mass of 79.91g/mol and a melting point of 3990C. Beryllium Chloride's chemical bonding is explored by writing down its Lewis structure using the Lewis method.
Following the Lewis structure, there is a requirement to comprehend its molecular geometry and the hybridization of the core element, Beryllium. To comprehend the MO diagram of beryllium chloride, the molecular orbital (MO) theory will be applied.
As far as electron and molecular geometry is concerned, they are both linear. Since the central Be atom has no lone pair and two bonding atoms (group / domains), the electron-pair and molecular geometry of BeCl2 is linear and the bond angle is \ [{180^0} ]. The electrons in an atom's outermost shell are depicted in the Lewis structure of any molecule. These electrons will have both bonding and non-bonding properties.
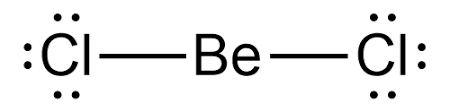
Lewis structure for BeCl2
Note:
Lewis’s structure is also known as electron dot structure or Lewis dot structure because the valence electrons in the molecule's Lewis structure are depicted as dots. It is a two-dimensional structure in which each atom in the molecule strives to complete its octet by sharing, acquiring, or losing electrons.
