Question
Question: What is the difference between tautomerism and metamerism? A) diethyl ether and propyl ether are m...
What is the difference between tautomerism and metamerism?
A) diethyl ether and propyl ether are metamers
B) Lactam and lactim are tautomers
C) Keto and enol form are tautomers
D) None of the above
Solution
We have to remember that the tautomerism can be seen in ketones and alcohols forming keto-enol tautomers also amine and imine forming Imine-amine tautomerism. They are two different types of isomerisms.
Complete step by step answer:
We have to remember that metamerism is the concept in which compounds have the same molecular formula but there is a difference in the number of Carbon atoms or alkyl groups.
-O-, -S- these are called metamers.
For example:
We need to know that the ethoxyethane and 1-methoxy propane, these both compounds will have same molecular formula i.e. C4H10O but difference in alkyl groups.
Ethoxyethane: CH3CH2OCH2CH3
1-methoxypropane: CH3OCH2CH2CH3
We must know that tautomerism is the concept that arises due to the 1,3 migration of Hydrogen atoms from one polyvalent atom to another within a molecule. There is equilibrium between these two isomers that are obtained from this migration which are then known as tautomers and the phenomenon termed as tautomerism.
For example:
Propanone and Prop-1-ene-2-ol are the pair of tautomers.
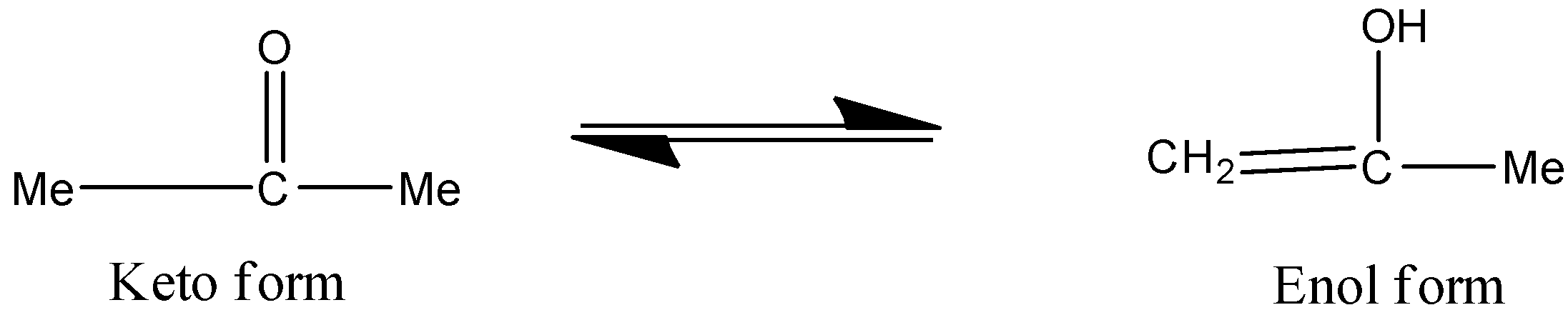
Looking at the options one by one:
Option A) this option can be correct as diethyl ether and propyl ether are metamers as explained above.
Option B) This option can be correct as this pair of compounds are tautomers.
Option C) this option can be correct as keto and enol forms are tautomers of each other as shown above.
Option D) this option is correct as all the given options are right as explained above.
Hence, the correct answer is, ‘Option D’.
Note: We also remember that metamerism is the concept in which compounds have the same molecular formula but there is a difference in the number of Carbon atoms or alkyl groups as is not the case with another kind of structural isomerisms.
