Question
Question: What is the difference between ethene, ethyne, ethylene, and acetylene?...
What is the difference between ethene, ethyne, ethylene, and acetylene?
Solution
Hint : Ethene, which is commonly known as ethylene signifies a compound containing two carbon that is connected with a double bond. Ethyne, which is commonly known as Acetylene, signifies a compound containing two carbons that are connected with a triple bond between them.
Complete Step By Step Answer:
Let us understand the given four compounds separately to understand the difference between them.
Ethene is a hydrocarbon, which is considered as per the rules of IUPAC, signifies eth− i.e. two carbon compounds and −ene i.e. double bond containing functional group alkene.
Hence, the chemical formula for ethene can be obtained by substituting the value of n (which shows the number of carbons i.e. n=2) in the general formula for alkene CnH2n
Thus the chemical formula for ethene is C2H4 and the structural formula is shown as
Ethyne is a hydrocarbon, which if considered as per the rules of IUPAC, signifies eth− i.e. two carbon compounds and −yne i.e. triple bond containing functional group alkyne.
Hence, the chemical formula for ethyne can be obtained by substituting the value of n (which shows the number of carbons i.e. n=2) in the general formula for alkyne CnH2n−2
Thus the chemical formula for ethyne is C2H2 and the structural formula is shown as
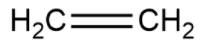
Ethylene is just another name used to define or signify ethene. It is a name formed by the common people for commercial use, without following any set of guidelines.
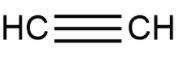
Acetylene is just another name used to define or signify ethyne. It is a name formed by the common people for commercial use, without following any set of guidelines.
Hence, there is no difference between Ethene and Ethylene. Similarly, there is no difference between Ethyne and Acetylene.
The difference between ethene and ethyne is the difference in the functional group or number of bonds between the carbon atoms, and the difference in chemical as well as physical properties due to the bonds.
Note :
We must note here that even though acetylene has a suffix −ene , it shows any alkyne compound. The reason for this exception is that this name is defined by or as per the rules of the IUPAC. This is a common name, made up and used by a community of people. Hence, it doesn’t follow IUPAC rules, or else its name would end with the suffix −yne as defined by the IUPAC i.e ethyne.
