Question
Question: What is the difference between a glycosidic linkage and peptide linkage?...
What is the difference between a glycosidic linkage and peptide linkage?
Solution
We will draw the structure of compound consisting of glycosidic linkage and peptide linkage respectively and analyze the difference between both the linkage in terms of groups attached as both have different linkage functional group and reaction through which they are formed.
Complete step by step answer:
| Glycosidic bond | Peptide linkage |
|---|---|
| A glycosidic bond or linkage is a type of covalent bond that joins a carbohydrate or sugar molecule to other group, which may or may not be different carbohydrate. | We know that a single amino acid has two ends, one with a carboxylic acid group and another with an amine group. When either of these groups attach with the opposite group of other amino acids an amide is formed with loss of water, this amide type of bond between two amino acids is called peptide bond. |
| A glycosidic bond is usually formed between the hemiacetal or hemiketal group of any of the saccharide (or a molecule that is derived from a saccharide) and the hydroxyl group of some compound . A substance that contain a glycosidic bond is called glycoside. | Peptide bond is formed in between carboxyl group and amino group. |
An example of glycosidic linkage is 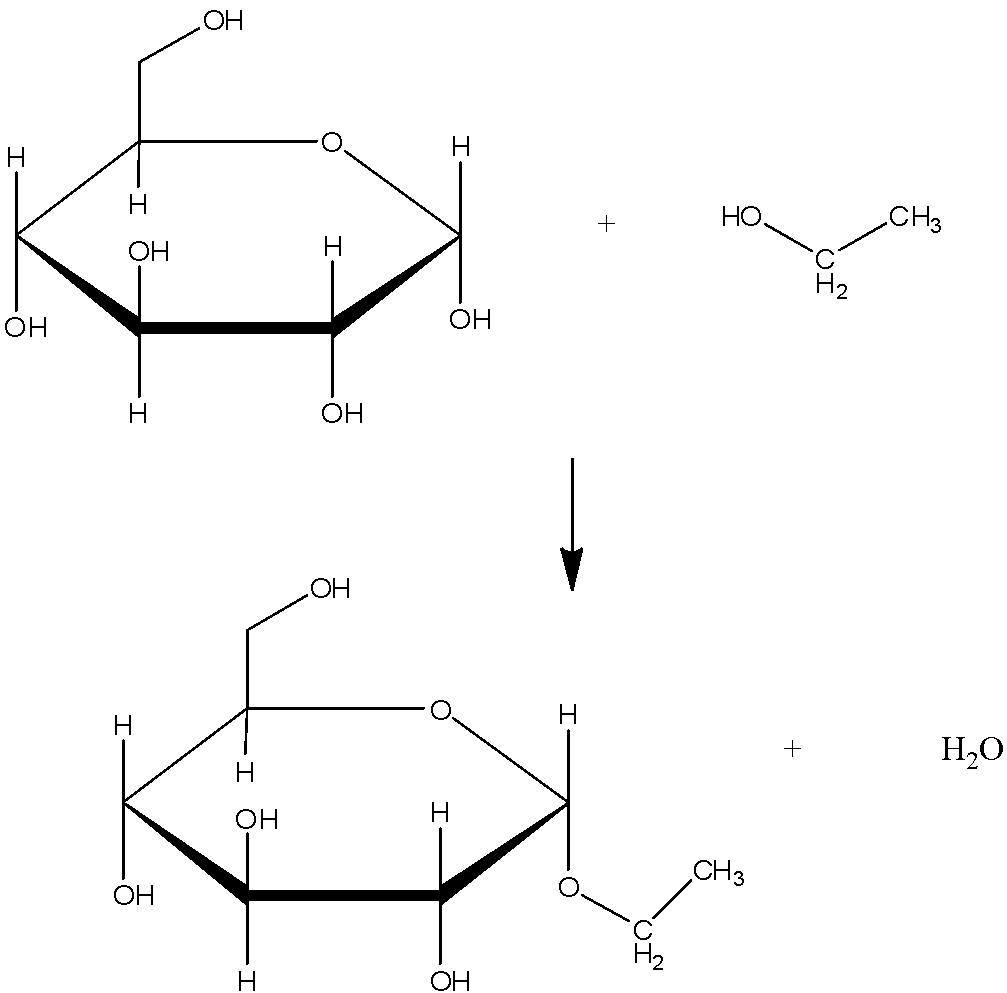 | The example of peptide bond is 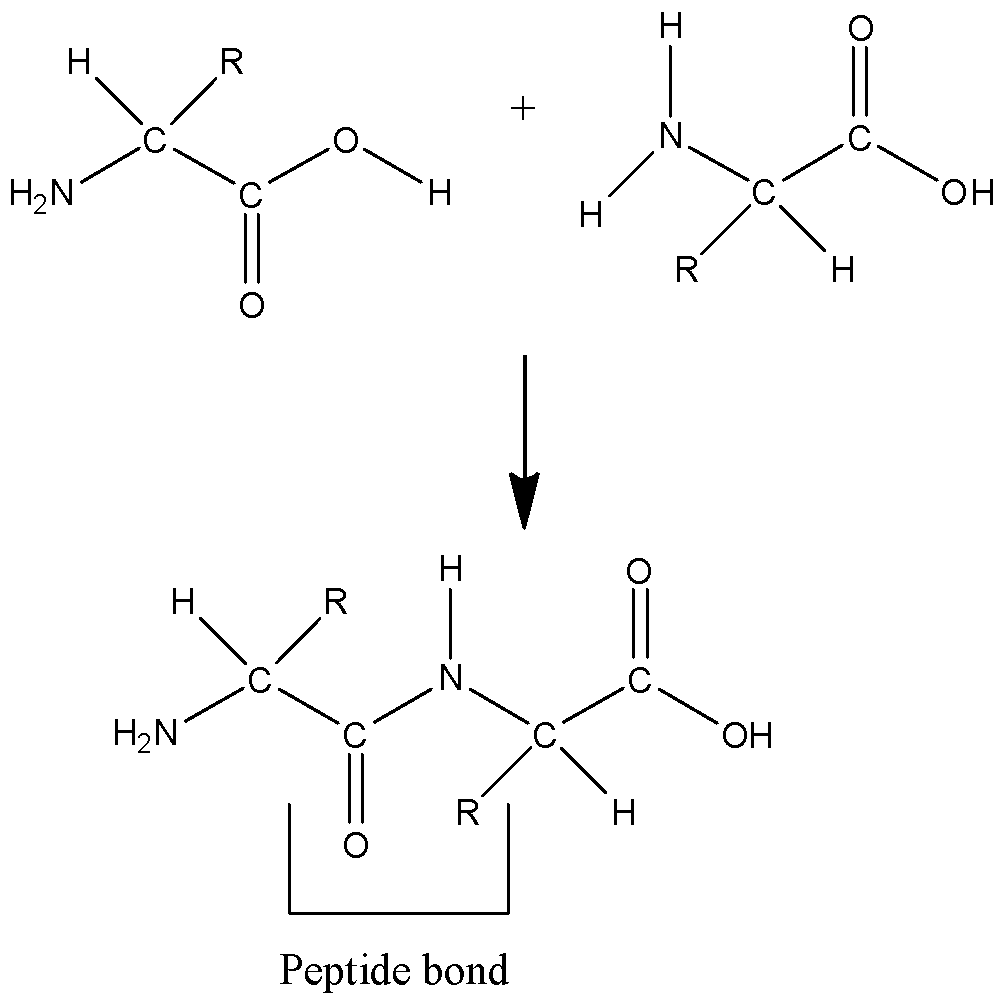 |
Additional information:
The term 'glycoside' also covers compounds in which the bonds are formed between hemiacetal (or the hemiketal) group of sugars and several chemical groups other than alcoholic groups, such as -SR (thioglycosides), -SeR (selena glycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides).
Particularly in naturally available glycosides, the compound ROH from which the carbohydrate residue has been removed is termed the aglycone, and the carbohydrate residue is sometimes called the 'glycine'.
Energy is given for formation of peptide bonds and by hydrolysis the peptide bond can be broken also. When a peptide bond breaks it releases energy. Due to the fact that peptides are resonance stabilized so they are stable and less reactive, lesser than esters also.
Note: There are two types of glycosidic bonds present α and β - glycosidic bonds. We can differentiate between them by their stereochemistry on the anomeric carbon. An α−glycosidic bond is formed when the attaching carbon have similar stereochemistry, whereas a β−glycosidic bond forms when the attaching carbon have different stereochemistry.
