Question
Question: What is the degree of unsaturation of cubane? (A) \( 6 \) (B) \( 2 \) (C) \( 8 \) (D) \( ...
What is the degree of unsaturation of cubane?
(A) 6
(B) 2
(C) 8
(D) 5
Solution
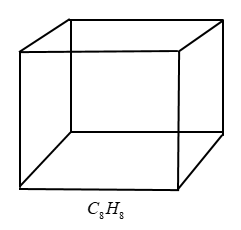
Cubane( C8H8C8H8 ) appears to possess six rings, corresponding to the six faces of a cube. However, in the case of hydrocarbons, you can use a simple trick- count the deficiency of hydrogens as compared to saturated open chain alkanes for a given number of carbons and then DBE is half of this deficiency.
Complete answer:
DBE or double bond equivalent a.k.a LU or level of unsaturation is the number of unsaturation’s present in an organic molecule. The term unsaturated means a double bond or a ring system. For instance, in benzene there are 3 double bonds and 1 ring which gives us 4 DBE. Moreover a triple bond can be regarded as DBE=2.
Cubane (C8H8C8H8) appears to possess six rings, corresponding to the six faces of a cube. However, in the case of hydrocarbons, you can use a simple trick- count the deficiency of hydrogens as compared to saturated open chain alkanes for a given number of carbons and then DBE is half of this deficiency.
Comparing with octane with molecular formula
C8H18
Deficiency of H in cubane compared to octane
=18−8
⇒10
Degree of unsaturation
=210=5
Hence the correct answer is option (D).
Additional Information:
It must be noted that X is the total number of halogens, namely Cl,Br,F and I present in the structure. Presence of an oxygen atom does not affect the DBE calculation.
Note:
The degree of unsaturation determines the double bond equivalent. So it should be done accordingly. DBE or double bond equivalent LU or level of unsaturation is the number of unsaturation’s present in an organic molecule. The term unsaturated means a double bond or a ring system.
