Question
Question: What is the decreasing order of stability of the following carbocation? A.\(m - (HO) - {C_6}{H_4}...
What is the decreasing order of stability of the following carbocation?
A.m−(HO)−C6H4−C+H2
B.p−(HO)−C6H4−C+H2
C.p−(CHO)−C6H4−C+H2
D.C6H5−C+H2
(1) B>A>D>C
(2) B>D>A>C
(3) B>D>C>A
(4) D>C>B>A
Solution
We have to remember that in carbocations, the compounds present in different positions determine the stability of the compound as a whole. To determine the stability of a chemical structure we have to check for the compound present differently in each structure whether it is an Electron Donating or an Electron withdrawing group.
Complete answer:
We will draw the chemical structures of each compound
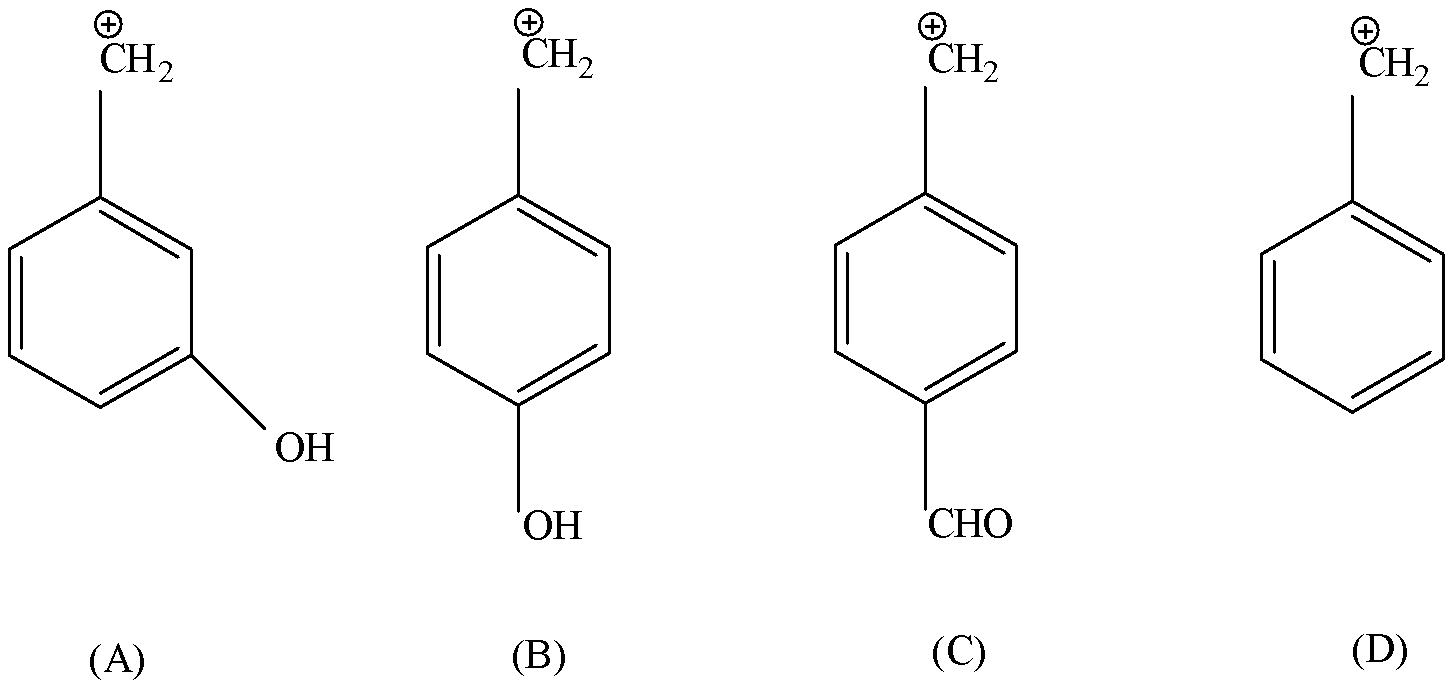
Here, in all the structures the CH2 cation is placed in all the structures at the same position.
Whereas there are two more compounds present – one is OH and another is CHO.
Let’s consider Structure B and C.
Now, OH shows +R and -I effect both, but here +R is dominant hence +R>-I. Hence OH is an Electron donating group.
Consider CHO, it shows -R and -I effect both, hence it is an Electron Withdrawing group. Since, the electron donating group shows higher stability then B is the highest stable and C is least stable as it is an electron withdrawing group.
So here we have,
B>−>−>C
Let’s consider structure A,
Here we have a group present at meta position, which will show inductive effect.
It implies, it will show -I effect and act as Electron withdrawing group which will decrease the stability of the overall compound.
In the structure D, there is no electron donating group as well as no electron withdrawing group. Hence is more stable than structure A but less stable than structure B.
Hence, the decreasing order of stability is
B>D>A>C
Hence, the correct answer is (2).
Note:
In cyclic compounds, we should first determine whether there are electronic donating/withdrawing groups present in the structures. As it plays an important role in determining the stability of a compound. Electronic donating group increases the stability of a compound and Electron withdrawing group decreases the stability of a compound. The groups present at para position are more stable than those present in meta position. As groups present on meta position show inductive effect only.
