Question
Question: What is the decreasing order of bond angles in \(B{{F}_{3}}\), \(N{{H}_{3}}\), \(P{{F}_{3}}\), and \...
What is the decreasing order of bond angles in BF3, NH3, PF3, and I3− ?
A. BF3 > I3− > PF3 > NH3
B. I3− > NH3 > PF3 > BF3
C. BF3 > NH3 > PF3 > I3−
D. I3− > BF3 > NH3 > PF3
Solution
Think about the hybridization, 3 dimensional orientation, and number of lone pairs on each central atom to identify the basic bond angle of the structure and then move on to how the lone pairs will affect the bond angle.
Complete step by step answer:
First, let us look at the geometry of all the molecules:
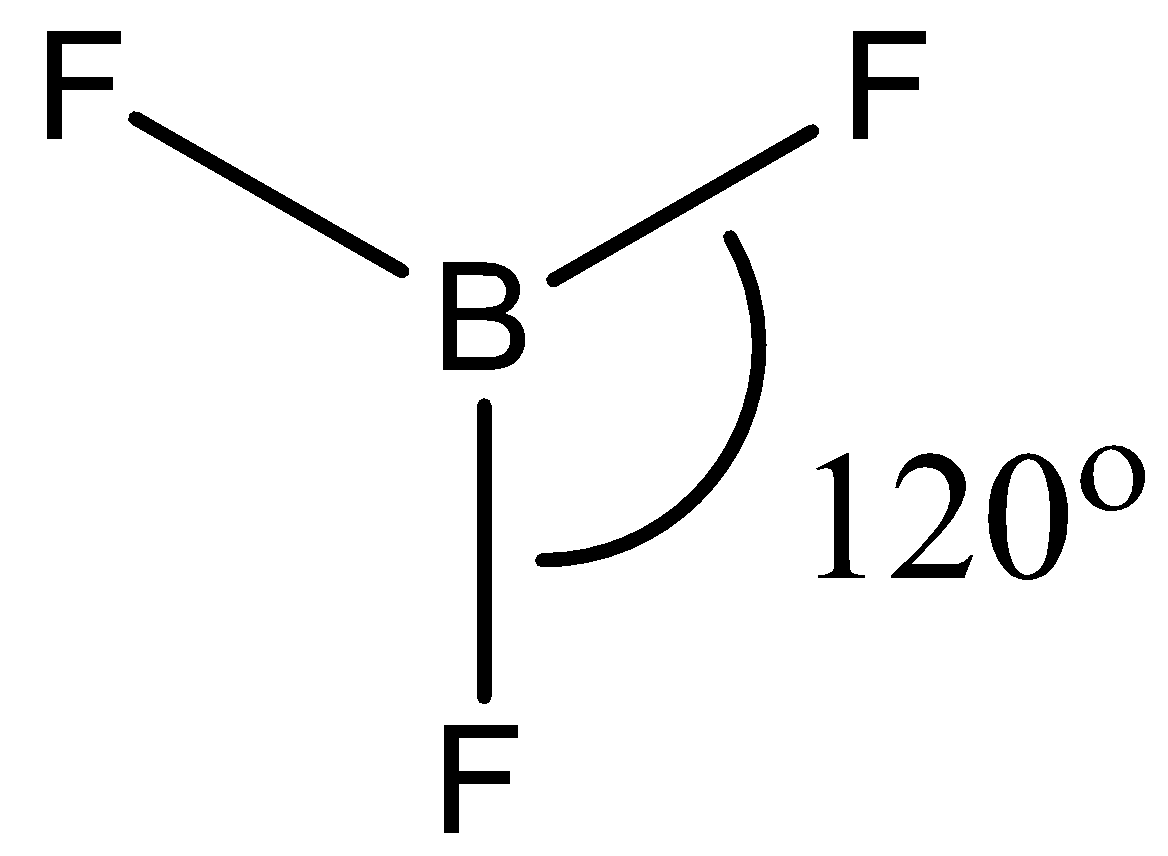
- The geometry of BF3 is trigonal planar. It involves 4 atoms, no lone pairs with a hybridization of sp2. This means that the bond angle between the atoms will be 120∘.
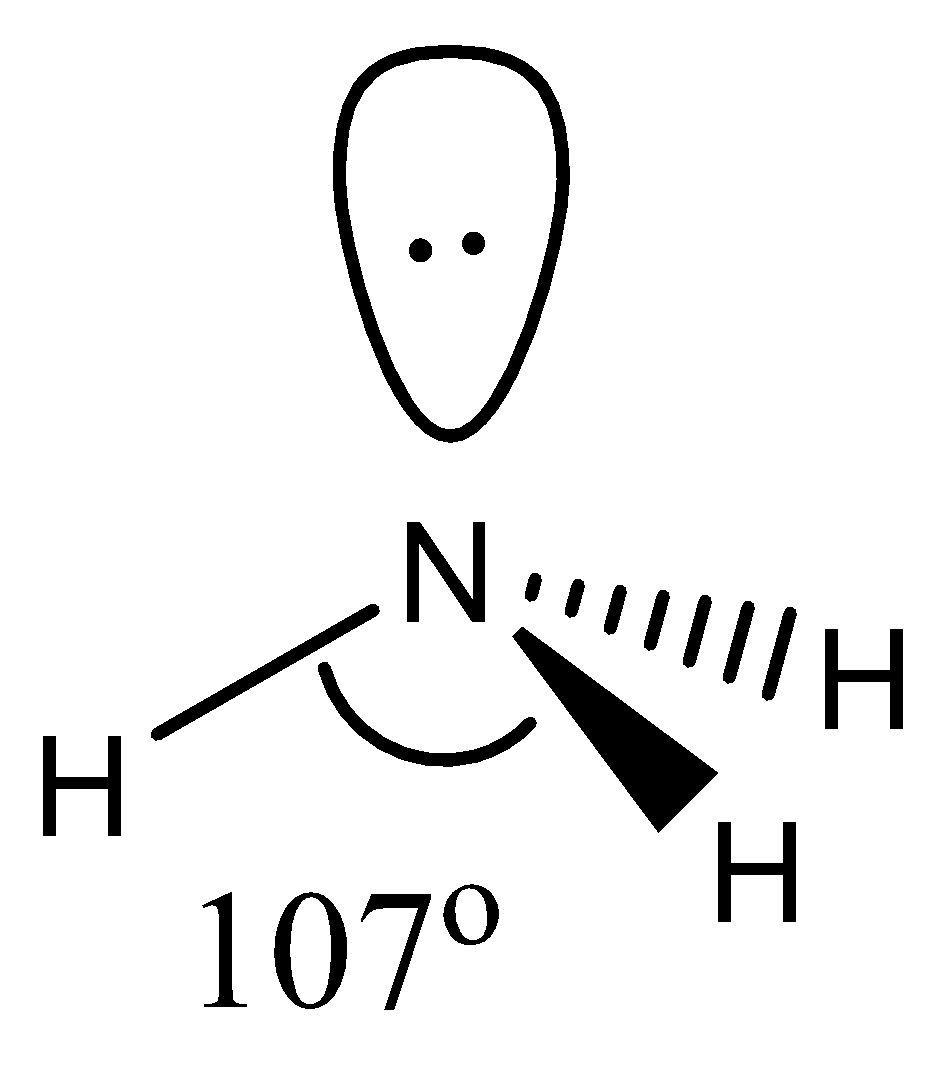
- The geometry of NH3 is pyramidal. Technically, the geometry is tetrahedral, but since one of the corners is occupied by a lone pair it is sometimes called pyramidal. It involves 4 atoms, 1 lone pair with a hybridization of sp3. This means that the bond angle will be less than 109∘ due to the presence of a lone pair. It is found to be around 107∘.
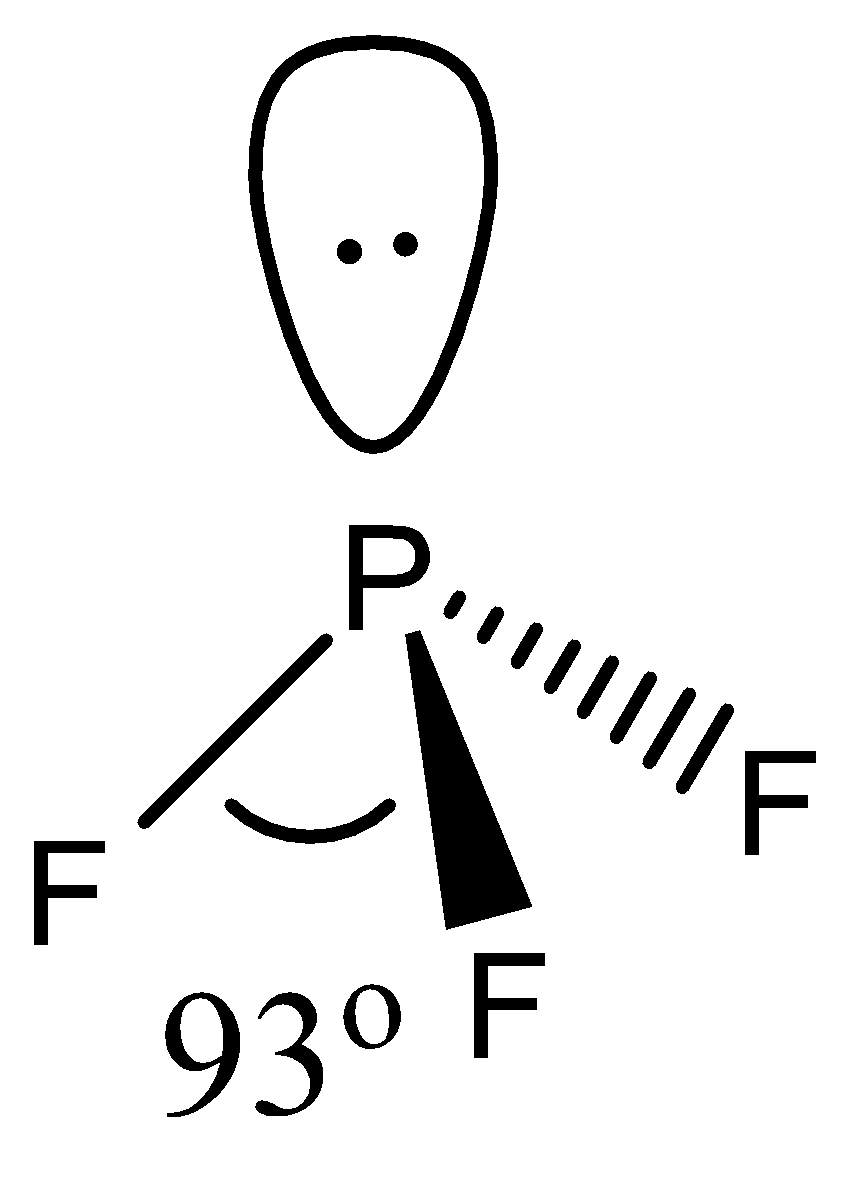
- The geometry of PF3 is again tetrahedral. It involves 4 atoms, 1 lone pair with a hybridization of sp3.
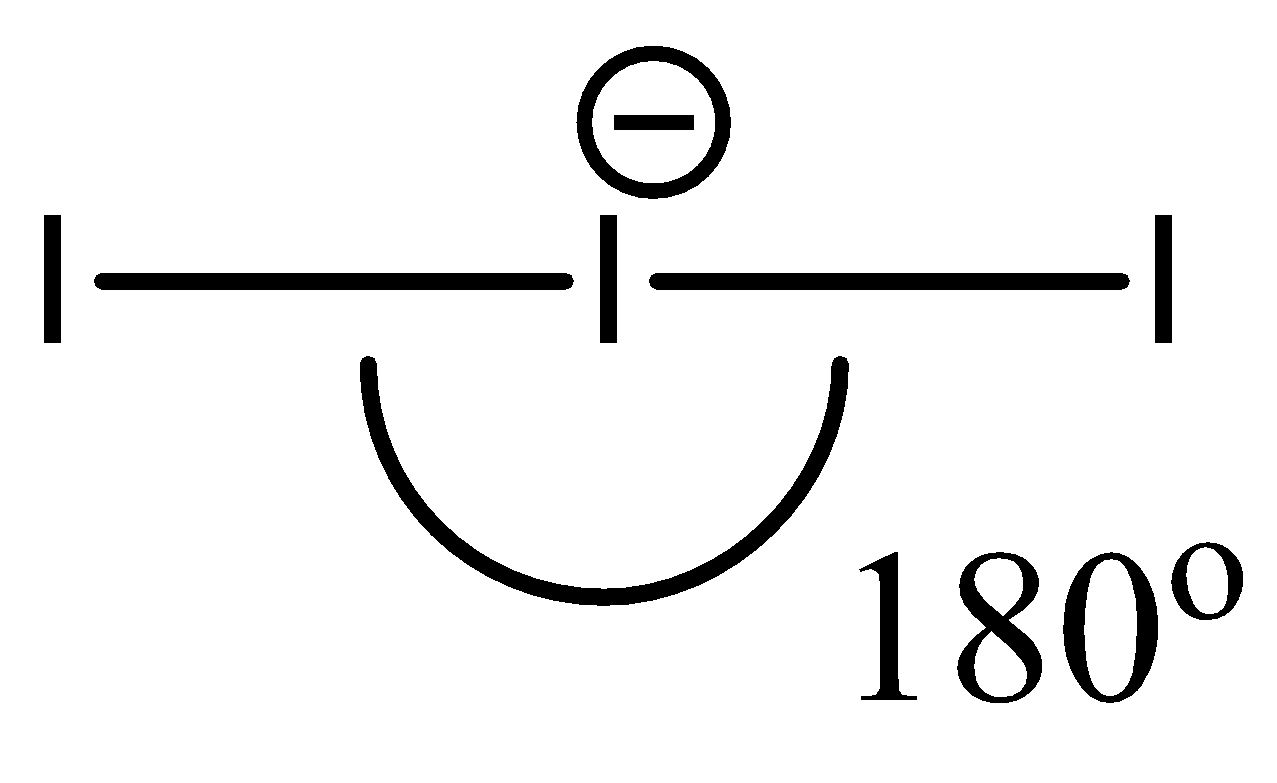
- Since I3− contains only 3 atoms, despite having an extra electron, its geometry is linear. Hence, the bond angle is around 180∘.
Hence, the answer is ‘D. I3− > BF3 > NH3 > PF3’.
Note:
The bond angle of PF3 is even smaller than NH3. This happens due to the larger size of F as compared to H. F also repels the lone pair present on P more strongly than Hdue to its own 3 lone pairs.
