Question
Question: What is the covalency of nitrogen in \({N_2}{O_5}\) ?...
What is the covalency of nitrogen in N2O5 ?
Solution
Draw the structure of nitrogen pentoxide. Nitrogen pentoxide contains two nitrogen atoms. Analyze the number of electrons a single nitrogen atom shares with other atoms in forming the bond.
Complete step-by-step answer: First let us understand what is covalency. Covalency comes into picture when we talk about atoms making bonds with the same or other atoms.
Covalent bonds are the bonds which are formed between two atoms by sharing electrons in the bond to attain electronic stability. Covalency is the number of atoms shared by an atom in the covalent bond to attain stable electronic configuration. For example if an atom shares one atom in the covalent bond its covalency is one. If it shares two electrons its covalency is two.
For seeing the covalency of an atom in a compound we should look at the number of bonds it is forming from its structure. So for finding covalency of nitrogen, let us look into the structure of N2O5:
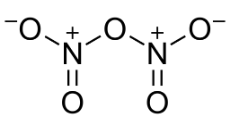
If we look at any of the two nitrogen atoms in nitrogen pentoxide, we can clearly see nitrogen is forming two types of bonds. Two single bonds and one double bond. One nitrogen atom forms a bond with three oxygen atoms. We know each covalent bond contains one pair of shared electrons. So one single bond between oxygen and nitrogen will consist of one electron from oxygen and one atom from nitrogen.
So one nitrogen atom shares four electrons via all the bonds it makes with neighboring oxygen atoms. It shares two electrons via two single bonds with two different oxygen atoms and it shares two electrons via a double bond with the third oxygen atom.
So, the covalency of nitrogen in nitrogen pentoxide is 4 .
Note: Keep in mind that nitrogen has five valence electrons but due to the geometry and bond formation of nitrogen in nitrogen pentoxide it shares only four electrons. Thus each nitrogen atom has a positive charge. So do not mistake in marking the covalency of nitrogen as 5 instead draw structure and analyze.
