Question
Question: What is the covalency of nitrogen in \({{N}_{2}}{{O}_{5}}\)?...
What is the covalency of nitrogen in N2O5?
Solution
We first need to know about what is covalency and the structure of N2O5 to reach the answer. Compounds containing two elements (so called binary compounds) can either have ionic or covalent bonding
Complete answer
In N2O5 we can see Nitrogen and Oxygen atoms are present which, if you look at the periodic table, you can see that they are both nonmetals. In between 2 non-metals there are always covalent bonds. So irrespective of whether it is N-N, N-O or O=O is being formed, the bond is always covalent.
If a compound is created from a metal and a nonmetal, its bonding is going to be ionic. If a compound is created from two non-metals, its bonding is going to be covalent.
N2O5 could be formed at a relatively low temperatures, like 60−800C, within 3−5 s when O3/NO is greater than 1.0. There was no N2O5 detected when the temperature was more than 1300C due to the decomposition of NO3. The proposed mechanism could give a good prediction of the experimental results along with kinetic simulation. Now let's come to the solution
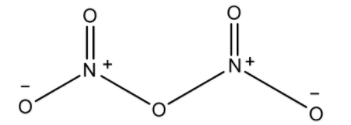
Covalency of Nitrogen in N2O5 is 4. Covalency is defined as the valence characterized by the sharing of electrons in a chemical compound.
The covalency of nitrogen in dinitrogen pentoxide is 4 because there are 3 covalent bonds and one coordinate covalent bond.
Note: N2O5 is used as a robust oxidizer in high-fuel rockets. It is also used in solvents that are not based on water, so that those molecules which are very sensitive to water can be easily nitrated. It can also be used as a nitrating agent in modern synthetic organic chemistry.
