Question
Question: What is the correct structure of the alkane 4-ethyl-2,2-dimethyloctane? A)  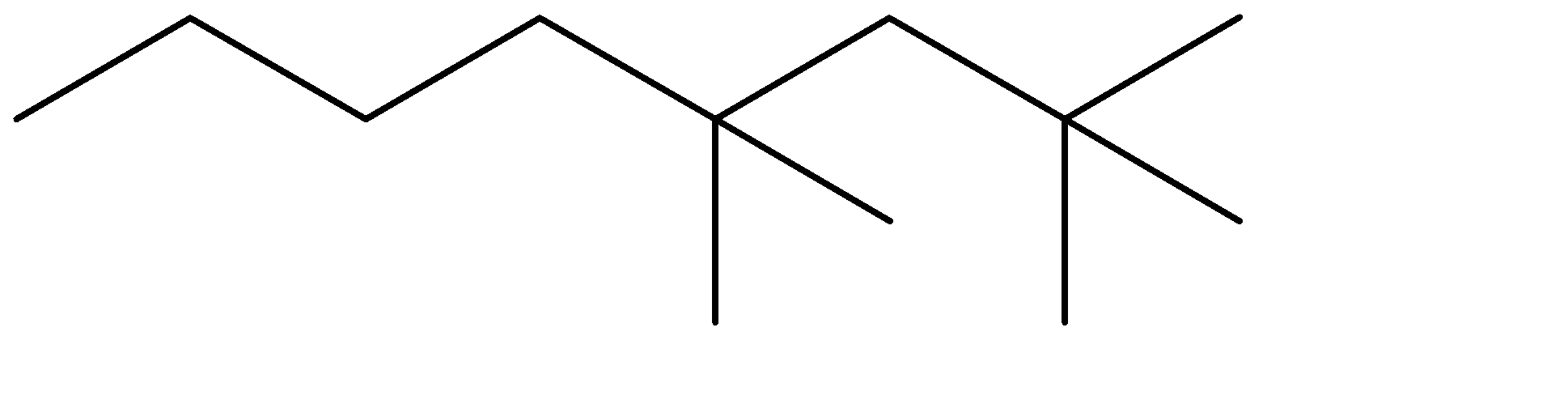
B) 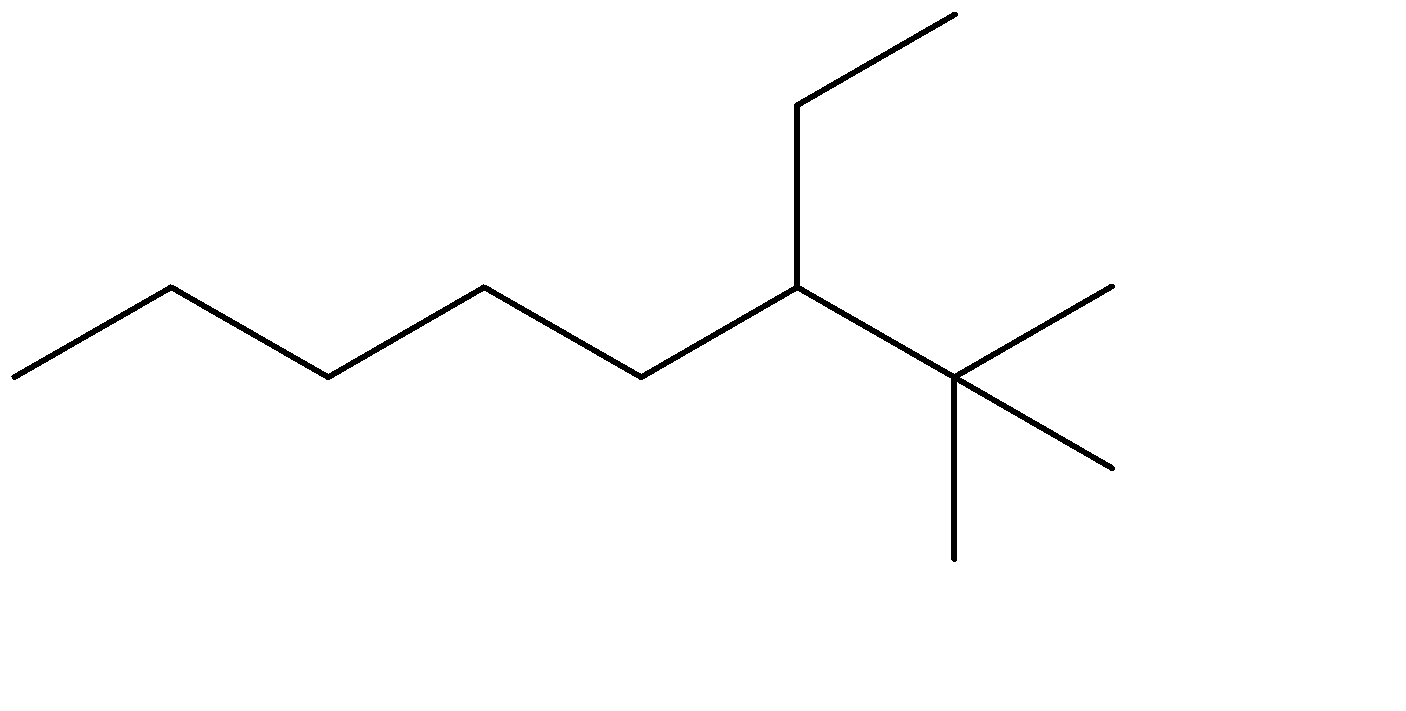
C)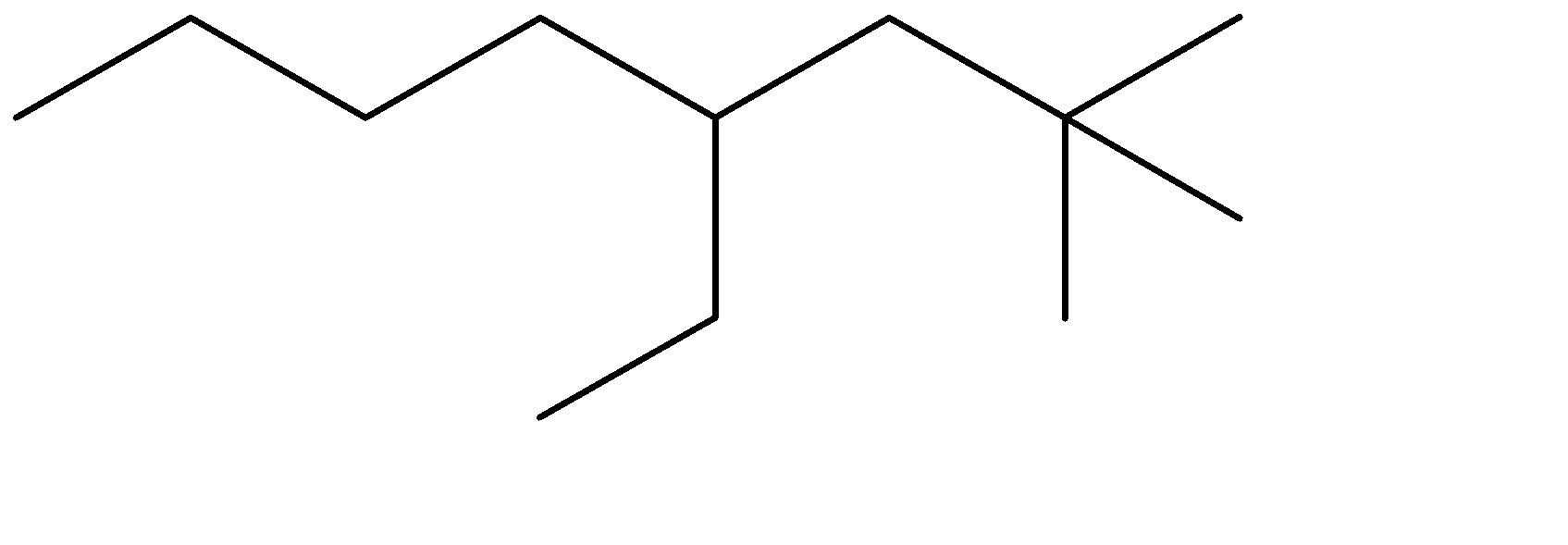
D) 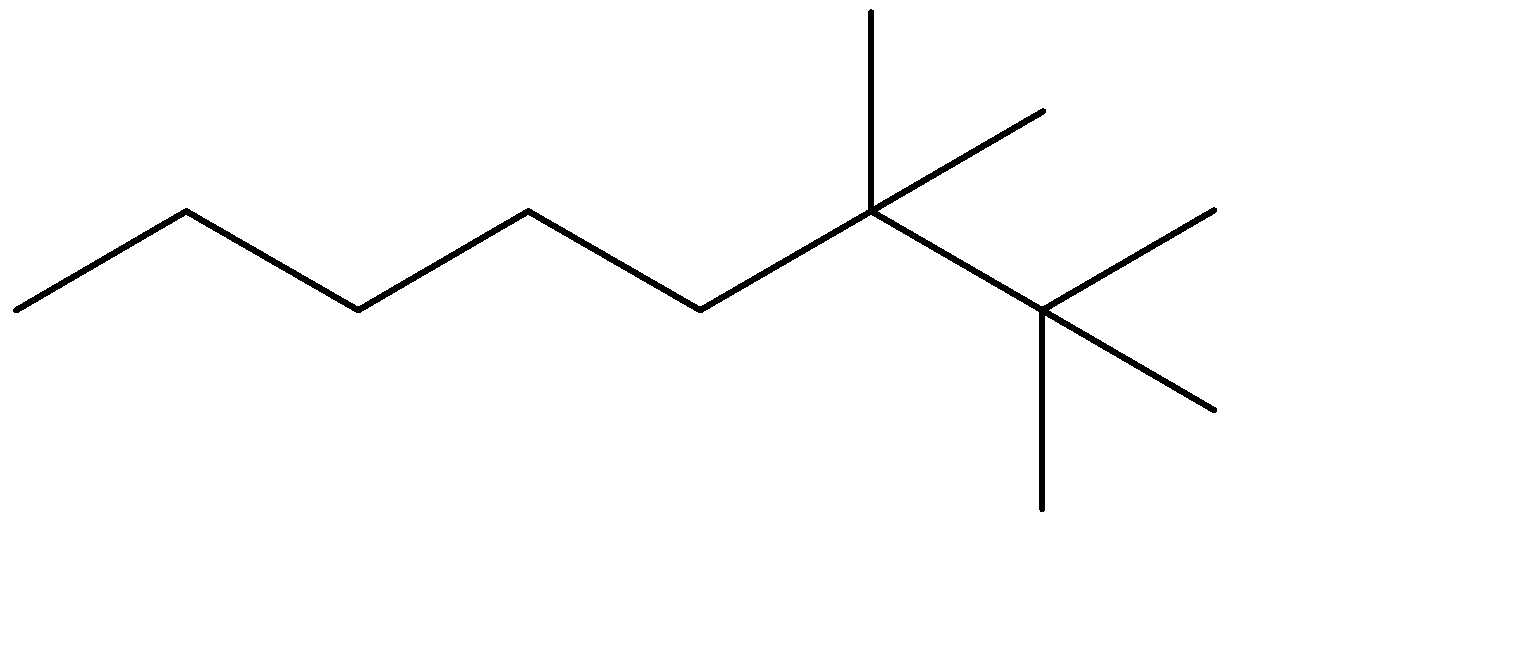
Solution
We need to solve this question with the help of IUPAC Nomenclature of compounds. Due to the discoveries of the quantity of organic compounds, there was a need for systematic nomenclature of compounds, and discard the vernacular nomenclature which led to high inconvenience. The parent chain is the chain having the longest no. of carbon atoms. The substituents attached to the parent chain are numbered such that the substituents receive the lowest number.
Complete Step By Step Answer:
The compound given to us is 4-ethyl-2,2-dimethyloctane. The compound is an alkane which means it is saturated and doesn’t contain any double bonds. The parent chain is Octane- meaning alkane with 8 carbon atoms. Out of the given options, all four have 8 carbon chains as their parent chain.
The substituents attached to the chain are ethane (ethyl is used when ethane is attached as an substituent) and 2 methyl groups. The numbers 4 and 2 determine the position of the substituents starting from either end of the parent chain.
4-ethyl, means ethyl substituent is located at the fourth position from one of the ends of the parent chain, from where the substituents can acquire minimum numbering. This condition is satisfied in Option (C) . The prefixes di, tri, tetra are used to determine the no. of same substituent attached as the substituent. Dimethyl means there are two methyl groups, and 2,2 determines that both the methyl groups are on the same carbon atom no. 2. 2 methyl groups at the C-2 position are satisfied in all four options. The correct structure is:
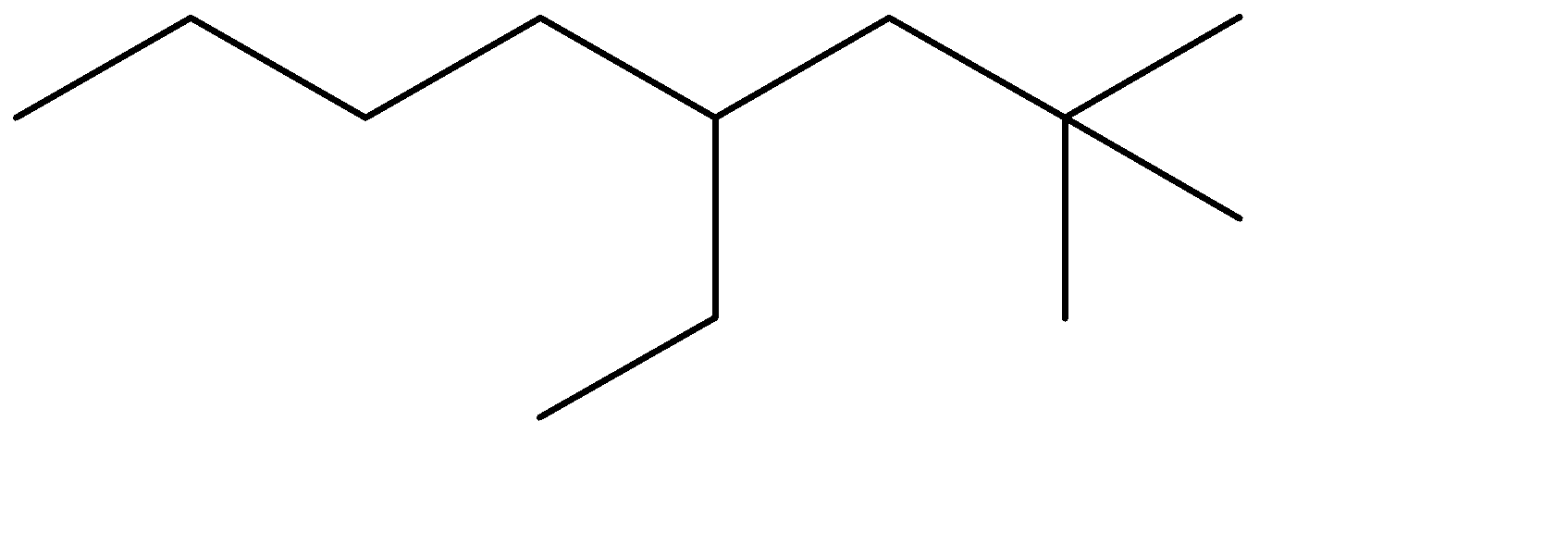
The correct option is therefore Option (C).
Note:
According to IUPAC :
1. The longest chain is the parent chain
2. the substituents attached are given the minimum numbering
3. In presence of various substituents the nomenclature is done alphabetically.
4. If other elements like Oxygen, Nitrogen, Sulphur are present, they will be termed as functional groups.
