Question
Question: What is the correct statement among the following about the structure of a borax molecule? A. all ...
What is the correct statement among the following about the structure of a borax molecule?
A. all the four boron atoms are sp3 hybridized.
B. all the four boron atoms are sp2 hybridized .
C. two of the four boron atoms are sp3 hybridized and the other two are sp2 hybridized.
D. Out of the 10 water molecules generally shown as water of crystallization in its MF.4 molecules of H2O are really involved in the structural formation.
Solution
Borax (Na2B4O7.10H2O) which is also known as Sodium Ortho Tetraborate.
It occurs as a tincal in dried-up lakes of India, Ceylon, Tibet, and California. Tincal is dissolved in boiling water and solution filtered to remove insoluble impurities such as sand, clay, etc. The resulting solution is concentrated to get crystals of borax.
From Boric acid: - Small quantities of borax are obtained from boric acid by neutralizing it with soda ash.
4H3BO3+Na2CO3→Na2B4O7+6H2O+CO2
It is a crystalline solid which is sparingly soluble in cold water but is readily soluble in hot water.
Complete step by step answer:
The ionic structure of borax is
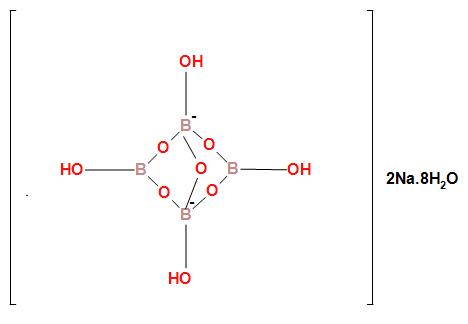
In the structure of borax, we can see that in the borate radical there are two different types of boron atom. The two boron atoms which are negatively charged and bonded tetrahedral fashion is sp3 hybridized. The other two boron atoms which are neural and bonded in trigonal planar fashion is sp2 hybridized. Therefore in borax two boron atoms are sp3 hybridized and two boron atoms are sp2 hybridized.
After discussing we conclude that the third statement is true about borax molecules.
So, the correct answer is Option C.
Note: Uses of Borax.
1.Borax is used as a flux for soldering and welding.
2.It is used in making enamels and glazes.
3.It is used for the stiffening of candle wicks.
4.It is used in borax bead tests for the detection of colored metal salts.
5.It is used as an antiseptic.
Borax is known in three forms.
Prismatic borax: - It is the common form and is the decahydrate form (Na2B4O7.10H2O) . It is obtained by crystallizing the solution at ordinary temperatures. It is less soluble in cold water but is soluble in hot water.
Octahedral form: - It is pentahydrate form (Na2B4O7.5H2O). It is obtained by the crystallizing solution at60∘C . It is Jeweller’s Borax.
Borax glass: - It is the anhydrous form (Na2B4O7). It is obtained by heating the common form above its melting point. It is a colorless glassy mass which is not stable in moist air. It gradually absorbs moist air and is converted into decahydrate form.
