Question
Question: What is the CORRECT order of reactivity of the following compounds in atomic nucleophilic substituti...
What is the CORRECT order of reactivity of the following compounds in atomic nucleophilic substitution reaction when treated with aq. NaOH ?
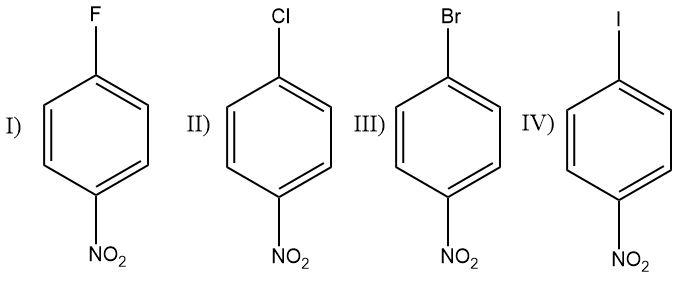
(A) I>II>III>IV
(B) IV>III>II>I
(C) II>III>I>IV
(D) IV>II>I>III
Solution
Sodium hydroxide is a strong base as well as a good nucleophile. The hydroxide ions released by sodium hydroxide attack the electron deficient nitro substituted benzene rings. The nucleophiles prefer position and rings with minimum electron density and therefore the electronegativity of halogens plays an important role in deciding the order.
Complete Step By Step Answer:
Nitro groups are strongly electron withdrawing in nature and therefore nitro substituted benzene rings show a higher reactivity towards the nucleophilic substitution reactions. Nucleophiles are electron rich species that attack cations or electron deficient groups.
The halogens placed at para positions to the nitro group in the benzene ring can further enhance the rate at which the nucleophile attacks the benzene rings. Halogens are electronegative species that have a tendency to pull the shared pair of electrons towards themselves. Halogens attached to the benzene ring act as electron withdrawing groups and pull the electron density of the ring towards themselves which makes the ring more sensitive towards a nucleophilic attack.
The size of the halogens increases down the group and the electronegativity decreases. Therefore fluorine being the first member of the halogen family has the highest electronegativity and therefore the highest tendency to act as an electron withdrawing group that pulls the electrons towards itself.
Iodine is the least electronegative atom and has minimum impact as an electron withdrawing group.
Therefore, the fluorine substituted ring has the highest reactivity towards nucleophiles, then chlorine, then bromine and lastly iodine substituted ring.
The correct order is (a) I>II>III>IV .
Note:
Benzene rings generally show electrophilic substitution reactions due to their electron rich nature, it is only due to the presence of halogens and nitro groups that the electron density shifts away from the benzene ring and allows a nucleophile to attack.
