Question
Question: What is the correct order of reactivity of alcohols in the following reaction? \[{\text{R - OH + H...
What is the correct order of reactivity of alcohols in the following reaction?
R - OH + HCl ZnCl2R - Cl + H2O
A. 1o ⟩2o ⟩3o
B. 1o ⟨2o ⟨3o
C. 3o ⟩2o ⟩1o
D. 3o ⟩1o ⟩2o
Solution
We use Lucas test to distinguish between primary, secondary and tertiary alcohols. In this test a solution of anhydrous zinc chloride in concentrated hydrochloric acid is used. This solution is commonly referred to as the Lucas reagent.
Complete step-by-step answer:
We must know that primary, secondary and tertiary alcohols are distinguished based on their reactivity with the Lucas reagent. In this reaction, an alcohol react with a hydrogen halide for exampleHCl, a nucleophilic substitution occurs, producing an alkyl halide and water in the presence ofZnCl2. As a by-product we get a chloride in the zinc-chloride bond being replaced by hydroxyl group from the given alcohol.
Now, we need to understand the mechanism of the reaction, in order to comment on the reactivity of the alcohol.
This reaction follows a SN1nucleophilic substitution mechanism which is a two-step process.
Step 1:- The hydroxyl group of the alcohol is protonated by the hydrochloric acid. Thus, making it a good leaving group resulting in the formation of carbocation. Now, in this reaction zinc chloride is used. It is a Lewis acid, forms a complex with the alcohol through association with an unshared pair of electrons on the oxygen atom. This enhances the hydroxyl’s leaving group potential sufficiently so that chlorine can displace it.
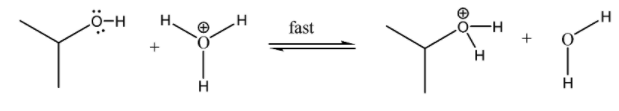
In this step, the alcohol accepts a proton.
The protonated hydroxyl group departs as a leaving group to form a carbocation and water molecule.
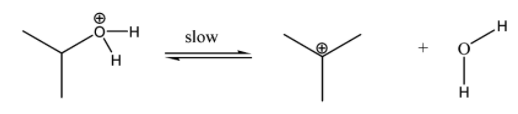
Step 2:- Lastly, the chloride ion attacks the carbocation and forms an alkyl chloride. This alkyl chloride is insoluble and hence turns the solution turbid.
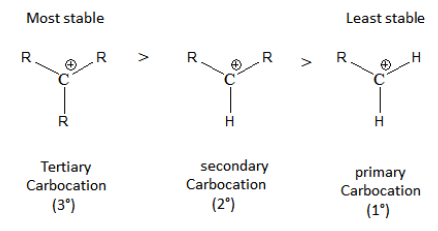
Now, the rate of formation of turbidity is observed fastest in the case of tertiary alcohol, as its carbocation is most stabilized by inductive effect and hyperconjugation effect, followed by secondary and lastly primary alcohol.
Tertiary alcohol →Solution turns turbid immediately
Secondary alcohol →Solution turns turbid after 3 to 5 minutes
Tertiary alcohol →Solution turns turbid only on heating
Hence, we can conclude that the correct answer is option C (3o ⟩2o ⟩1o).
Note:
1. We can also distinguish primary, secondary and tertiary alcohols by Victor Meyer's method.
2. In this method, the given alcohol is converted into an iodide by treating it with concentrated hydro iodic acid or red phosphorus and iodine. In the next step, the iodide is treated with silver nitrite to form nitro alkane.
3. In this reaction, 10 alcohols produced red colour, 20 alcohols generated blue colour, while in the case of 30 alcohols no colour was observed.
