Question
Question: What is the correct order of rate of nitration of the following compounds? \[\begin{aligned} &...
What is the correct order of rate of nitration of the following compounds?
& {{C}_{6}}{{H}_{5}}C{{H}_{3}}\text{, }{{C}_{6}}{{H}_{6}}\text{, }{{C}_{6}}{{D}_{6}}\text{, }{{C}_{6}}{{T}_{6}}\text{, }{{C}_{6}}{{H}_{5}}B{{r}_{3}}\text{, }{{C}_{6}}{{H}_{5}}N{{R}_{3}}\text{, }{{C}_{6}}{{H}_{5}}NM{{e}_{2}} \\\ & \text{ A B C D E F G } \\\ \end{aligned}$$ A.G > A > B > C > D > E > F B.G > B > C > D > A > F C.G > A > B = C = D > E > F D.G > A > B > C = D > F > ESolution
Nitration is an electrophilic substitution reaction. Here, electrophile is, which reacts with the benzene ring. Rate of nitration is found to be directly proportional to the stability of carbocation intermediate.
Complete answer:
- The correct order of nitration will be G > A > B = C = D > E > F, let’s discuss it in detail:
- G.
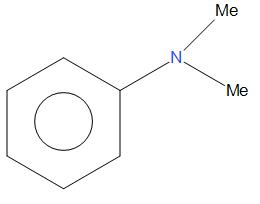
Here, we can say that C6H5NMe2 is most stable and will show highest rate of nitration as compared to all other given compounds. This is because there is a strong electron donating group present that will show more +I effect and +R effect, that is there will be more electron density in the ring, due to which it undergoes nitration at a faster rate.
- A.
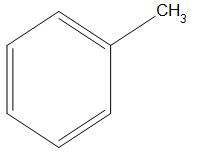
Here, we can see that an electron donating group is present that is CH3 . Which will show +I effect (it is an effect which is shown by those species which have a tendency to donate electrons, present in the carbon chain, and the charge is relayed through the chain and this effect is called +I effect) and will push the electrons. Hence, we can say it will have rate of nitration less than that of C6H5NMe2
- B. C6H6
- C. C6D6
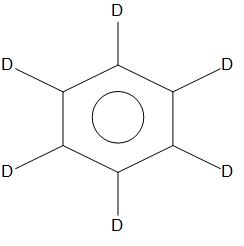
- D. C6T6
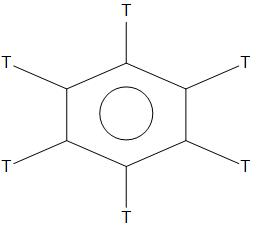
In C6H6,C6D6,C6T6,we can see that there is no +I or +R effect. As there is no side chain is present, hence it is found to have less rate of nitration than that of C6H5NMe2and C6H5CH3. Hence, in these three compounds the rate of nitration will be equal.
- E. In C6H6Br3, there is a halogen Br is present, which will show -I and +R effect. Here, it is found that the +R effect is more dominant. Hence, it will have rate of nitration less than that of C6H5CH3
- F. C6H5NR3:
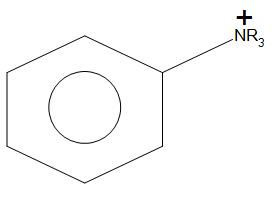
The rate of nitration will be minimal as compared to all other given compounds. This is because it will show –I and –R effect (due to –I effect the electron withdrawing group will start withdrawing electrons from the adjacent carbon atom, and hence this will be no electrons available for donation, due to which rate of nitration will be less) due to which it will destabilise the carbocation.
- Hence, we can conclude that the correct option is (C), that is the correct order of rate of nitration of the given compounds is G > A > B = C = D > E > F.
Note:
- We can say that the electron donating groups in compounds will increase the rate of nitration.
- Compounds that will show + I effect will show the highest rate of nitration. Due to the +I effect the electron donating group will start donating electrons, and hence this will be more electrons available for donation, due to which rate of nitration will be more.
