Question
Question: What is the correct name of \(I{{F}_{7}}\). (A) Iron heptafluoride. (B) Iodine heptafluoride ...
What is the correct name of IF7.
(A) Iron heptafluoride.
(B) Iodine heptafluoride
(C) Iodine septafluroide
(D) Iron (IV) septafluoride.
Solution
IF7 is the best example for interhalogen compounds. Interhalogen compounds mean halogens themselves react to each other and form interhalogen compounds. IF7 has a structure of pentagonal bipyramidal.
Complete step by step solution:
-In IF7, one iodine and seven fluorine atoms are present.
-The chemical name of IF7 is Iodine heptafluoride.
-In the chemical name of IF7 the name of Iodine should be written first because of its less electronegativity. Fluorine's name should be written later because it is more electronegativity.
-The prefix hepta in the name of IF7 - Iodine heptafluoride indicates the presence of seven fluorine atoms.
So, the correct option is (B).
Additional information:
-The structure of IF7 is as follows.
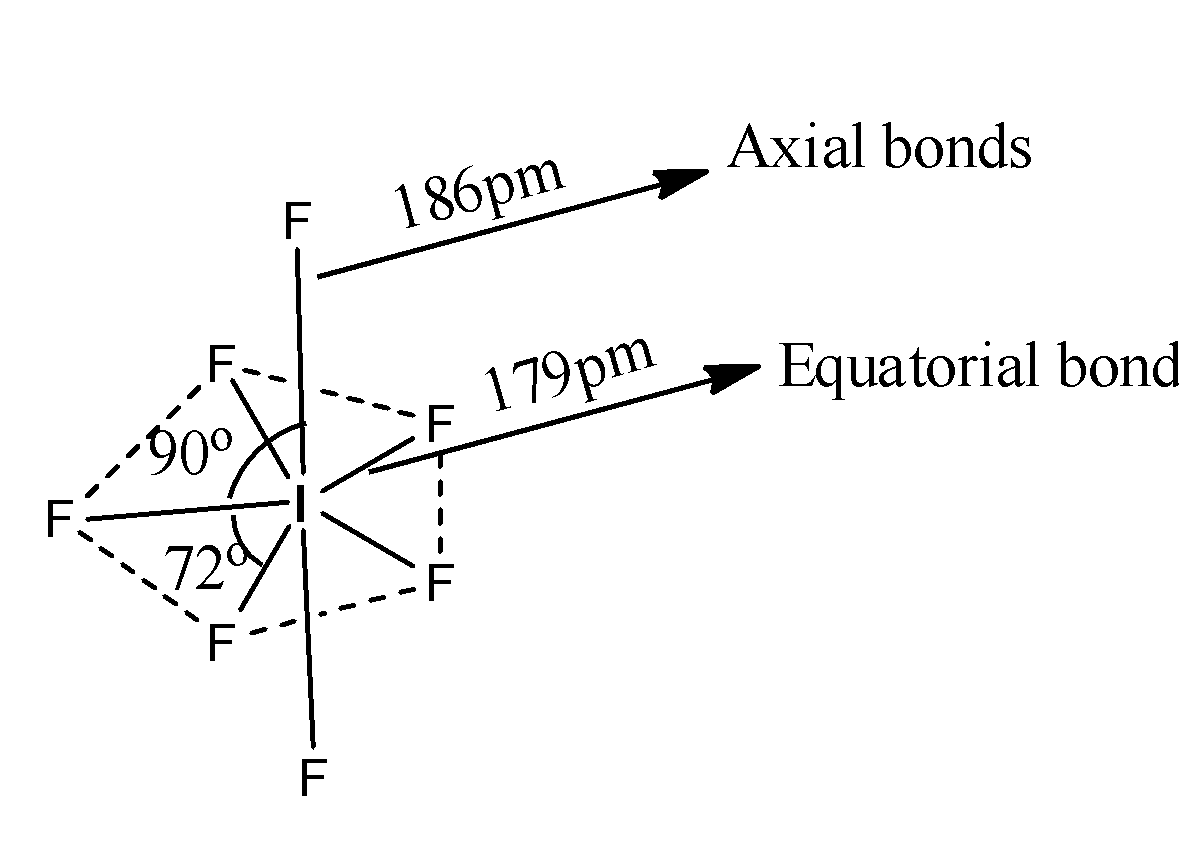
-The resultant geometry of IF7 is pentagonal bipyramidal.
-The hybridization of Iodine in IF7 is sp3d3 and the overlapping of fluorine with iodine orbitals we can see as follows.
-There are two types of bonds axial and equatorial bonds.
-Axial bonds are longer than equatorial bonds.
-There are two types of orthogonal angles, there are 90 and 72.
Note: If any chemical contains a number of repeating atoms in its chemical formula then we are not supposed to write the number in the chemical name. If the compound contains Two same atoms – di, three same atoms – tri, four same atoms – tetra, five same atoms – penta, six same atoms – hexa, seven same atoms – septa, eight same atoms – octa.
