Question
Question: What is the correct increasing order of bond lengths of bond indicated as I,II,III and IV in the fol...
What is the correct increasing order of bond lengths of bond indicated as I,II,III and IV in the following compounds?
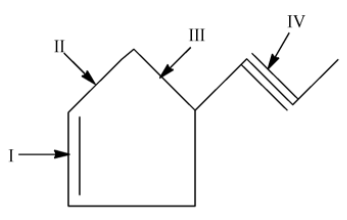
A. I < II < III < IV
B. II < III < IV < I
C. IV < II < III < I
D. IV < I < II < III
Solution
Carbon has a valency of four, and three types of bonds can be made by it. The bonds can be sp,sp2 and sp3hybridised. The bond lengths of the respective bonds can be accordingly determined.
Complete answer:
In order to answer our question, we need to know about the tetravalence of carbon and properties of double and triple bonds. Now, carbon has a valency of four, but all of them are not situated in one plane, in fact they are symmetrically distributed in space. The carbon atom may be supposed to be present in the centre of a regular tetrahedron and it’s four valencies directed towards its four corners at and angle 109028′ to each other. Whereas three valencies of carbon lie in one plane, the fourth one is directed behind the plane.
The carbon atoms involved in the formation of double bonds are sp2 hybridised. This means that three orbitals in the valence shell of the carbon atom in its excited state get hybridised to form three equivalent hybrid orbitals. The hybridisation is also called trigonal hybridisation. The double bond is made up of a sigma bond surrounded by two pi electrons.
In triple bonds, atoms are sp hybridised. Two orbitals in the valence shell of the carbon atom in its excited state get hybridised to form two equivalent hybrid orbitals. Thus, a triple bond is made up of a sigma bond surrounded by two pi bonds involving four electrons.
Bond length follows the fashion: single bond > double bond > triple bond. It is because the larger the number of electrons shared by the two atoms, greater the attractive force between the electrons and nuclei, lesser the bond strength.
Now, let us come to our question. From the relation given above, we can say that IV < I . III is shorter than II as it is close to the triple bond. But III and II are single bonds and they have more bond length.
So, the final order is: IV < I < II < III, which gives the correct answer to be option D.
Note:
In organic or carbon compounds, s and p orbitals are involved. This leads to three types of hybridisation which are sp,sp2and sp3.
| Mixing atomic orbitals | Hybrid orbitals | Hybridisation |
|---|---|---|
| One s and three p | sp3 | sp3hybridisation |
| One s and two p | sp2 | sp2hybridisation |
| One s and one p | sp | sp hybridisation. |
