Question
Question: What is the correct formula for gold (III) Chloride? A) \(A{{u}_{2}}C{{l}_{3}}\) B) \(A{{u}_{3}}...
What is the correct formula for gold (III) Chloride?
A) Au2Cl3
B) Au3Cl
C) AuCl3
D) AuCl
Solution
Every chemical formula is written by crisscrossing the valency of the element.
- Notation of gold in periodic table is Au and for chlorine is Cl.
Complete step by step answer:
To solve these types of questions we need some pre-requirements i.e.
-We should know the chemical representation or chemical symbols of each element say,
For Sodium –Na
Potassium-K
Nitrogen-N
Silver –Ag
Chlorine – Cl
Fluorine –F etc.
-Here in this question the given compound is gold (III) chloride, foe gold the chemical symbol is Au and for chloride it’s Cl.
-So that part is done and now we have to know the charges assigned for these i.e. whether they are positively charged ions (cations) or negatively charged ion (anions).
-For determining the cation and anion there is an easy way, the first name in the compound will be of positive ion and the second name will be of negative ion.
- For example Sodium chloride (NaCl) - Here the first word sodium is positively charged ion Na+ and the second name chlorine is the negatively charged ion Cl−1
-In this case gold (III) chloride, gold is the cation and chloride is the anion.
And the number given in the parentheses is the oxidation state of the gold.
-Charge carried by the ion is a very important factor while writing the chemical formulae.
Gold has +3 charge and for Chlorine it is -1 charge so criss crossing these charges of the element we will get the chemical formula for the compound.
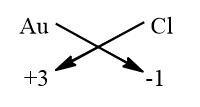
And we get the final formulae as AuCl3. The correct option is option “C” .
Note: While writing the formula by criss crossing the charges carried by the ions, we will only consider the numerical part. We will not take into account the sign, possessed by the ions whether it is negatively or positively charged.
