Question
Question: What is the correct electronic configuration of the central atom in \(K_{ 4 }[Fe(CN)_{ 6 }]\) on cry...
What is the correct electronic configuration of the central atom in K4[Fe(CN)6] on crystal field theory?
(A.) t2g4eg2
(B.) t2g6eg0
(C.) e3t23
(D.) e4t22
Solution
Hint : To find the correct answer first try to find the oxidation state of Fe in this complex and then write its electronic configuration. Here you have to remember that CN− is a strong ligand that would pair up the electrons present in the d-orbitals.
Complete step by step solution :
Let’s find the correct answer to this question,
First, we need to calculate the oxidation state of iron -
4(+1) + x + 6(-1) = 0
+4 + x -6 = 0
x = +2
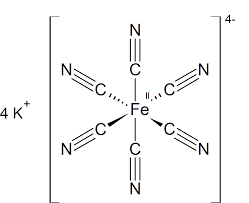
In K4[Fe(CN)6],
Electronic configuration of Fe in ground state = [Ar]3d64s2
Electronic configuration of Fe in excited state = Fe2+= [Ar]3d64s0
We can represent them as:
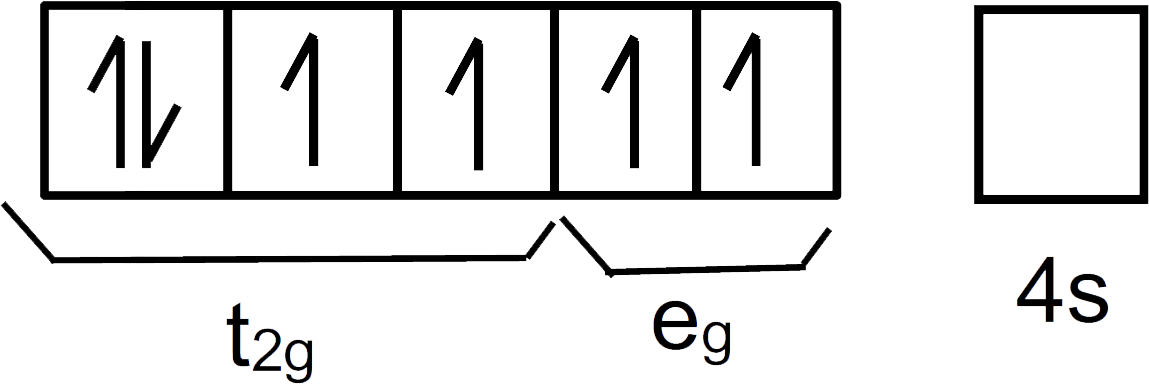
In this compound CN− is a strong field ligand that would pair up the electrons present in the d-orbitals.
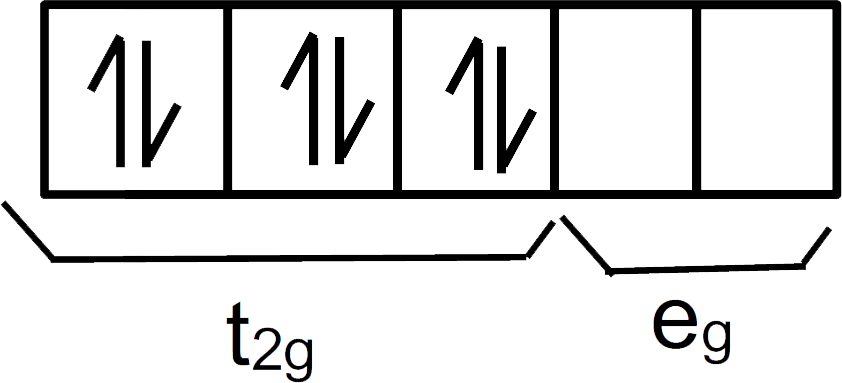
We can see there are 3 pairs of electrons in t2g and 0 pairs of electrons in eg.
So, the correct electronic configuration of the central atom in K4[Fe(CN)6] on crystal field theory is t2g6eg0
So, the correct answer is “Option B”.
Additional Information: t2g and eg refer to symmetry classes to which the d-orbitals belong in octahedral complexes.
In the crystal field model, the normally degenerate 3d orbitals split into two groups, a doubly degenerate set of two orbitals and lower energy triply degenerate set. When analyzed by mathematical group theory, the wave functions for these two groups fall into different symmetry groups, designated eg and t2g respectively.
Note : Solid potassium ferrocyanide, both as the hydrate and anhydrous salts, has a complicated polymeric structure. We can also draw the structure of this complex K4[Fe(CN)6] as,