Question
Question: What is the correct dipole moment of \(N{{H}_{3}}\) and \(N{{F}_{3}}\) respectively? (A)- \(4.90\t...
What is the correct dipole moment of NH3 and NF3 respectively?
(A)- 4.90×10−30 C m and 0.80×10−30 C m
(B)- 0.80×10−30 C m and 4.90×10−30 C m
(C)- 4.90×10−30 C m and 4.90×10−30 C m
(D)- 0.80×10−30 C m and 0.80×10−30 C m
Solution
Dipole moment is a measure of polarity of a bond. It is the product of the charges and the distance between partial charges. It is a vector quantity and its direction is always given from less electronegative atom to more electronegative atom.
It is generally expressed in debye (D) and 1 D = 3.33564×10−30 C m.
Dipole moment of polar molecules containing lone pairs is the vector sum of dipole of lone pair and net dipole moments of bonds.
Complete answer:
Both NH3 and NF3 have trigonal pyramidal shape.
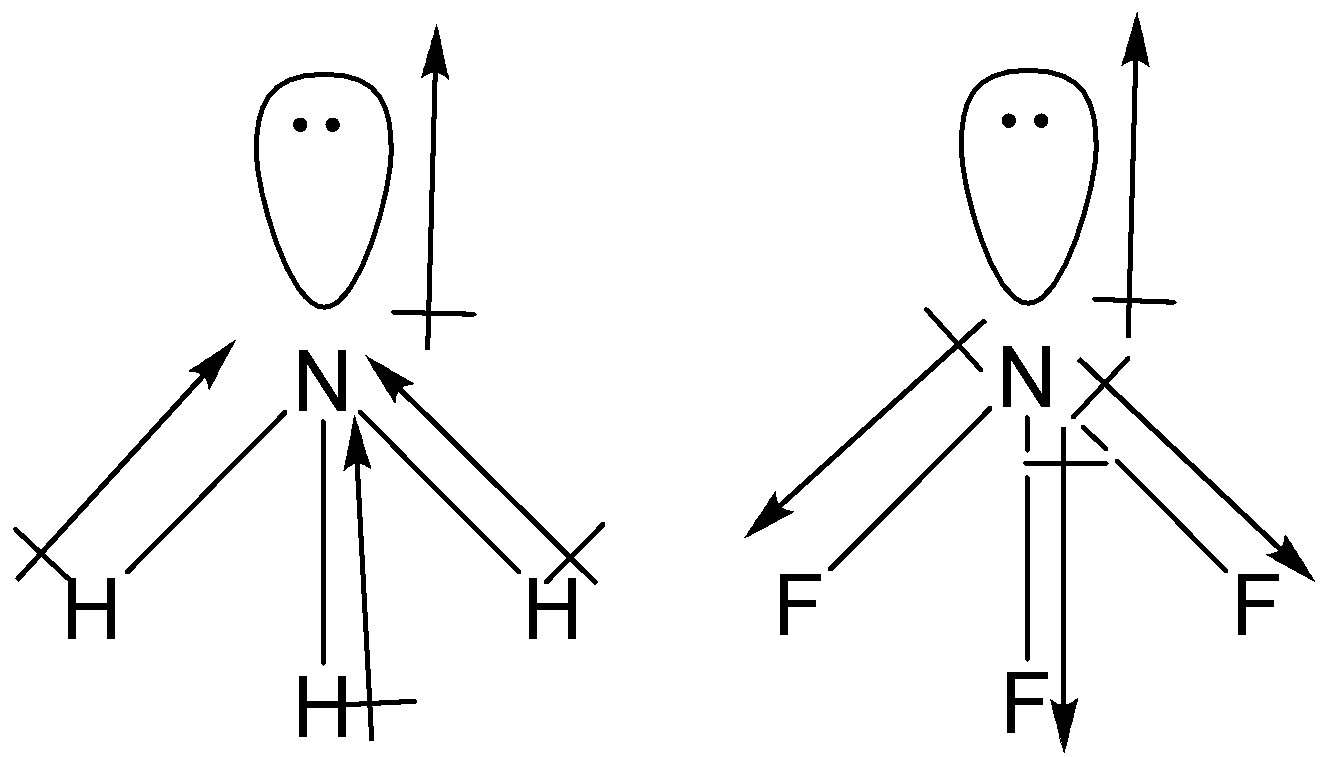
The dipole moment of lone pairs in NH3 and NF3 is away from nitrogen.
Dipole moment of NH3
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of N−Hbond will be form H to N. The net dipole moment of three N−H bond will add up to 1.4 D. As we know that 1 D = 3.33564×10−34 C m.
Then, 1.4 D will be equal to 1.4×3.33564×10−30 C m, i.e. 4.90×10−30 C m.
Dipole moment of NF3
Electronegativity of F is more than that of N, thus the direction of dipole moment of N−F bond will be from F to N. As we can see that the direction of N−F bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of NF3 has been found to be 0.24 D.
Multiplying 0.24 D with 3.33564×10−30 C m, we get the dipole moment of 0.80×10−30C m.
So, the correct answer is “Option A”.
Note: The dipole moment of lone pairs in NH3 and NF3 is away from nitrogen.
Dipole moment of NH3
We know that nitrogen is more electronegative than hydrogen. Therefore, the dipole moment of N−Hbond will be form H to N. The net dipole moment of three N−H bond will add up to 1.4 D. As we know that 1 D = 3.33564×10−34 C m.
Then, 1.4 D will be equal to 1.4×3.33564×10−30 C m, i.e. 4.90×10−30 C m.
Dipole moment of NF3
Electronegativity of F is more than that of N, thus the direction of dipole moment of N−F bond will be from F to N. As we can see that the direction of N−F bond is opposite to that of the lone pair on N atoms. So, the net dipole moment of NF3 has been found to be 0.24 D.
Multiplying 0.24 D with 3.33564×10−30 C m, we get the dipole moment of 0.80×10−30C m.
