Question
Question: What is the coordination number in a square close packed structure in two dimensions? A.\(2\) B...
What is the coordination number in a square close packed structure in two dimensions?
A.2
B.3
C.4
D.6
Solution
Coordination number is the number of atoms or ions that are immediately surrounding a central atom in a complex or crystal. As to find coordination numbers, we have to find the arrangement of square close packed structures in two dimensions.
Complete step by step answer:
In two dimensional close packing, the row of closed packed spheres are stacked to obtain a two dimensional pattern.
This close packing can be done in two ways.
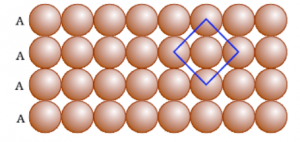
-AAA type- Here the sphere in the second row may be placed in a way such that they are touching each sphere of the first row and exactly above the sphere of the first row. In this type coordination number is 4
-ABA type- Here the sphere in the second row may be placed in the depression of the first row. This produces a different type row from the first type. In this type coordination number is 6
The packing fraction of the square lattice in two dimensions is the ratio between the area occupied by the circles and the total area.
Here, square close packing is that the second row of atoms can be placed exactly below the first row.
If we see in a square closed packed structure a molecule is in contact with four of its neighbours.
Hence, the coordination number in a square close packed structure in two dimensions will be four.
Therefore, Option C is correct.
The coordination number in a square close packed structure in two dimensions is 4.
Note:
Close packing can be defined as a packing of a sphere in such a way that the atoms occupy maximum available space and hence, there will be minimum empty space. As we can see in the above picture that every row is placed just exactly below the other that is known as square close packing.
