Question
Question: What is the common name of propanone? (A) Oxobutane (B) Acetone (C) Ethyl acetone (D) Ethyl ...
What is the common name of propanone?
(A) Oxobutane
(B) Acetone
(C) Ethyl acetone
(D) Ethyl methyl ketone
Solution
Hint:The organic compound with formula (CH3)2CO is acetone or propanone. It is the smallest ketone and the simplest. It is a colourless liquid that is highly volatile and flammable liquid which also has a pungent odour.
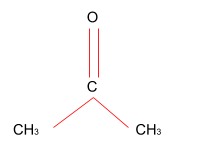
Complete step by step solution:
Acetone can dissolve many fats and resins, as well as cellulose ethers, acetate cellulose and nitrocellulose and other esters of cellulose. During the production of artificial fibres (such as a few rayons) and explosives, acetone is extensively employed because of its latter quality.
It is used in pharmaceuticals as chemical intermediate, solvent in vinyl and acrylic resins, lacquers, alkyd paints, inks, cosmetics and coats of varnish. It is used in paper coating, adhesives, and heat-screen coating preparation and is also used in the synthesis of many compounds as a starting material.
In commercial acetone production, the cumene hydroperoxide process is the dominant process. Dehydrogenation of 2-propanol (isopropyl alcohol) is also used for acetone.
Acetone is manufactured by normal metabolism in the human body and is disposed of in the same way. It is mostly present in blood and urine. It is produced in larger quantities by those with diabetes. Reproductive toxicity tests show that reproductive problems caused by it may be low.
Note: Acetone is manufactured from propylene directly or indirectly. The cumene process generates approximately 83 per cent of acetone; hence, the production of acetone is linked to the production of Phenol. Other processes involve direct oxidation of propylene or propylene hydration to oxidize (dehydrogenated) acetone with two-propanol.
