Question
Question: What is the common name of chlorophenyl methane? a.) Benzyl Chloride b.) Benzoic chloride c.) ...
What is the common name of chlorophenyl methane?
a.) Benzyl Chloride
b.) Benzoic chloride
c.) Benzylic pentachloride
d.) None of these
Solution
Benzyl indicates an alkyl group. Alkyl groups are obtained when hydrogen atoms of alkane are substituted. Chloro indicates the presence of one chlorine atom. In chlorophenyl methane, two hydrogen atoms are substituted, one with chlorine atom and other with phenyl group. In Acids, oic is usually used.
Complete step by step answer:
When hydrogen atom of benzene is removed, the Phenyl group is obtained which is represented as Ph or C6H5−.
Toluene is methyl benzene. When Hydrogen atoms of benzene are replaced by methyl group, Toluene or methyl benzene is obtained.
Toluene is C6H5−CH3 also known as phenylmethane. When a hydrogen atom of methyl group is replaced with Chlorine atom, chlorophenyl methane is obtained.
- •
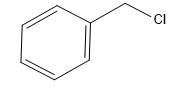
Chlorophenyl Methane can be represented as C6H5−CH2−Cl.
Methane is CH4, when two hydrogen atoms of methane, one with chloro atom and other with Phenyl group, then chlorophenyl methane is obtained.
Benzyl group is represented as C6H5−CH2− and common name for chloroalkane is Alkyl Chloride, so common name for chlorophenyl methane is Benzyl chloride.
-In carboxylic acid oic acid is used such as benzoic acid, benzoic chloride is not correct.
-benzylic pentachloride indicates five chlorine atoms, but chlorophenyl methane indicates one chlorine atom. In nomenclature, halogens and phenyl groups are always prefix substituents, hence Methane is considered as a parent chain. So, the correct answer is “Option A”.
Note: When two hydrogen atoms of methane, one with chloro atom and other with Phenyl group, then chlorophenyl methane is obtained. Benzyl group is represented as C6H5−CH2−. In nomenclature, halogens and phenyl groups are always prefix substituents, hence Methane is considered as a parent chain.
