Question
Question: What is the common name for chlorophenyl methane? A.Benzyl chloride B.Benzoic chloride C.Benzy...
What is the common name for chlorophenyl methane?
A.Benzyl chloride
B.Benzoic chloride
C.Benzylic pentachloride
D.None of the these
Solution
We have to know that Chlorophenyl Methane is an organochlorine compound that is highly reactionary. It appears to be colourless to slightly yellow liquid. It is widely used in the chemical building block.
Complete step by step answer:
We can give the structure of chlorophenyl methane as,
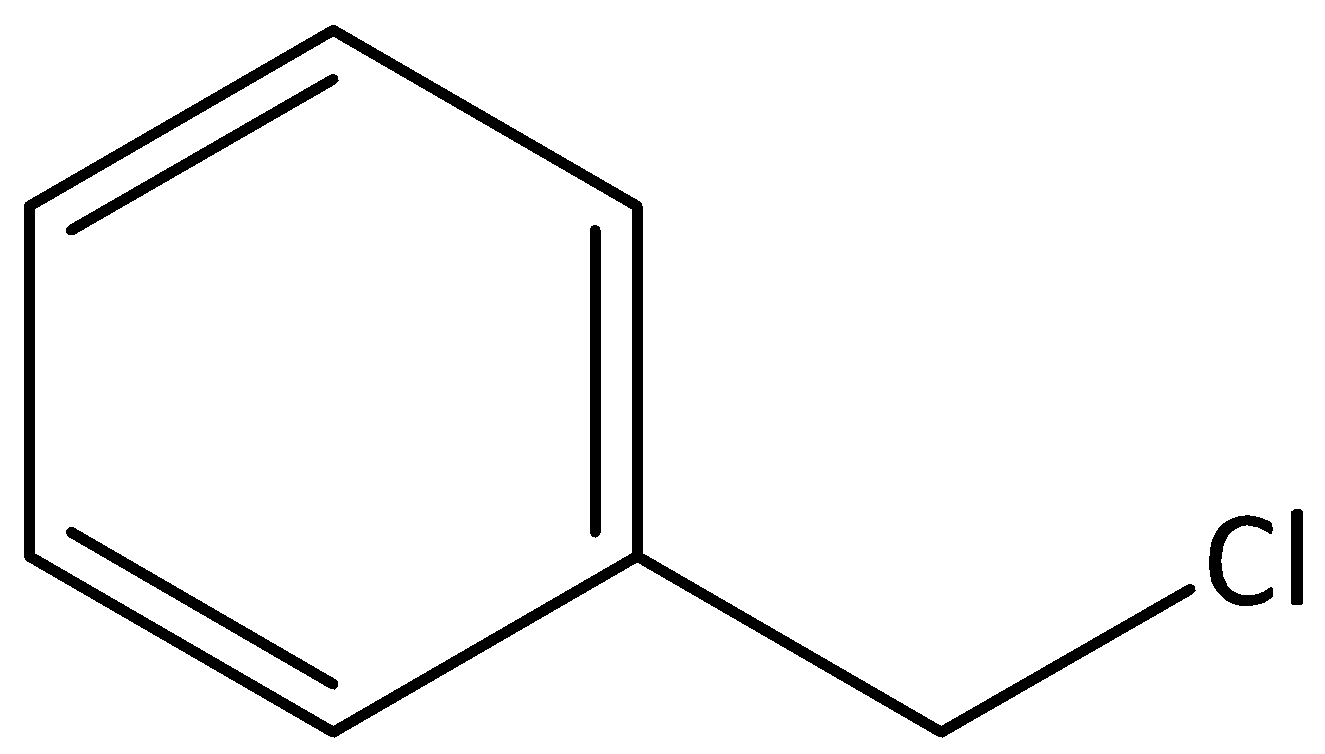
From the given structure, we can see that a methyl group and a chloro group are attached to a six membered ring that is benzene. The common name for chlorophenyl methane would be Benzyl chloride.
Therefore, option (A) is correct.
Benzyl chloride is otherwise known as α−chlorotoluene. The chemical formula of benzyl chloride is C6H5CH2Cl.. It has pungent odour and is mildly soluble in water, whereas highly soluble in ethanol, ethyl ether, chloroform, and miscible in organic solvents.
We can prepare benzyl chloride from Blanc chloromethylation of benzene.
Treatment of benzyl alcohol with hydrochloric acid would result in the formation of benzyl chloride.
Industrially, we can prepare benzyl chloride by the photochemical reaction of toluene with chlorine. We can write the chemical equation as,
C6H5CH3+Cl2C6H5CH2Cl+HCl
The reaction begins by the process of free radical, which involves the intermediacy of free atoms of chlorine. Benzal chloride and benzotrichloride are obtained as side products of the reaction.
When we oxidize the benzyl chloride in the presence of alkaline potassium permanganate, we get benzoic acid as the product. The chemical equation is written as,
C6H5Cl2+2KOH+2[O]C6H5COOH+KCl+H2O
Therefore, Option (A) is correct.
Note:
We can use benzyl chloride as the precursor for preparation of benzyl esters, which are used as plasticizers, flavorants, and perfumes. We can obtain dibenzyl ether when benzyl chloride is reacted with aqueous sodium hydroxide. Benzyl chloride could be used in the synthesis of amphetamine-class drugs. Benzyl chloride on reaction with metallic magnesium forms a Grignard reagent.
