Question
Question: What is the chemical formula of Acetylene? \(\begin{aligned} & a){{C}_{2}}{{H}_{4}} \\\ & ...
What is the chemical formula of Acetylene?
a)C2H4b)C2H2c)C2H6d)C3H8
Solution
Hint: Acetylene is a very widely used hydrocarbon and is the simplest alkyne found in nature. With this in mind, now try and work out what its chemical formula could possibly be.
Step-by-Step Solution:
Let us analyse acetylene as a chemical compound first, along with all its chemical and physical properties. This should help us answer this question very easily.
Acetylene, also called Ethyne, the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colourless, inflammable gas widely used as a fuel in oxyacetylene welding and cutting of metals and as raw material in the synthesis of many organic chemicals and plastics; its chemical formula is C2H2.
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with C-CH bond angles of 180°. The structure of acetylene is as follows:
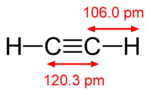
In terms of valence bond theory, in each carbon atom the 2s orbital hybridizes with one 2p orbital thus forming an sp hybrid. The other two 2p orbitals remain unhybridized. The two ends of the two sp hybrid orbital overlap to form a strong σ valence bond between the carbons, while on each of the other two ends hydrogen atoms attach also by σ bonds. The two unchanged 2p orbitals form a pair of weaker π bonds.
Therefore, per our analysis, the answer to this question is b).
Note: Approximately 20% of acetylene is supplied by the industrial gases industry for oxyacetylene gas welding and cutting due to the high temperature of the flame. Combustion of acetylene with oxygen produces a flame of over 3,600 K (3,330 °C; 6,020 °F), releasing 11.8 kJ/g. Oxyacetylene is the hottest burning common fuel gas.
