Question
Question: What is the chemical formula for the polyatomic ion acetate?...
What is the chemical formula for the polyatomic ion acetate?
Solution
Chemical formula is going to explain the proportions of the chemicals which are present in a particular compound. The chemical symbols of the elements which are present in a particular compound going to be represented in the chemical formula.
Complete answer:
- In the question it is asked to write the chemical formula for the polyatomic ion acetate.
- First we should know about the acetate ion before we are going to know about the polyatomic ion acetate.
- The chemical formula to represent the acetate ion is as follows.
CH3COOH⇌Acetate ionCH3COO−+H+
- Acetate anion is going to be generated from the acetic acid.
- Acetate anion also called as a conjugate base of acetic acid.
- The salt of the acetic acid is called acetate ion.
- Two moles of the acetic acid undergo condensation reaction and form a chemical called polyatomic ion acetate.
- The chemical reaction of converting acetic acid to polyatomic ion acetate is as follows.
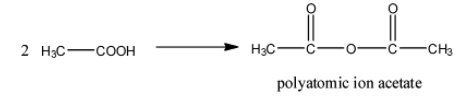
- From the above chemical reaction we can write the chemical formula of the polyatomic ion acetate.
- The chemical formula of polyatomic ion acetate is (CH3CO)O .
Note:
Acetate ions undergo condensation reaction and form polyatomic ion acetate and it is also called as acetic anhydride. Acetic anhydride has a big role in chemical reactions to do acetylation reactions to prepare acetylated products. In the synthesis of aspirin acetic anhydride has a crucial role.
