Question
Question: What is the chemical form for barium bromate?...
What is the chemical form for barium bromate?
Solution
Barium is a chemical element with the symbol 'Ba' and the atomic number of Ba is 56. Barium is not found in nature as a free element because of its high reactivity. Similarly, bromine is another element that has the symbol 'Br' with an atomic number 35 which is also highly reactive hence which is not found in nature as a free element.
Complete step by step answer:
We have to know that the chemical form of barium bromate is Ba(BrO3)2 with molecular mass, Barium bromate is a white crystalline powder or powder which is denser than water and it is faintly soluble in water. When it is reacted with organic compounds, it may explode and it is used as a corrosion inhibitor.
We can draw the structure of barium bromate as,
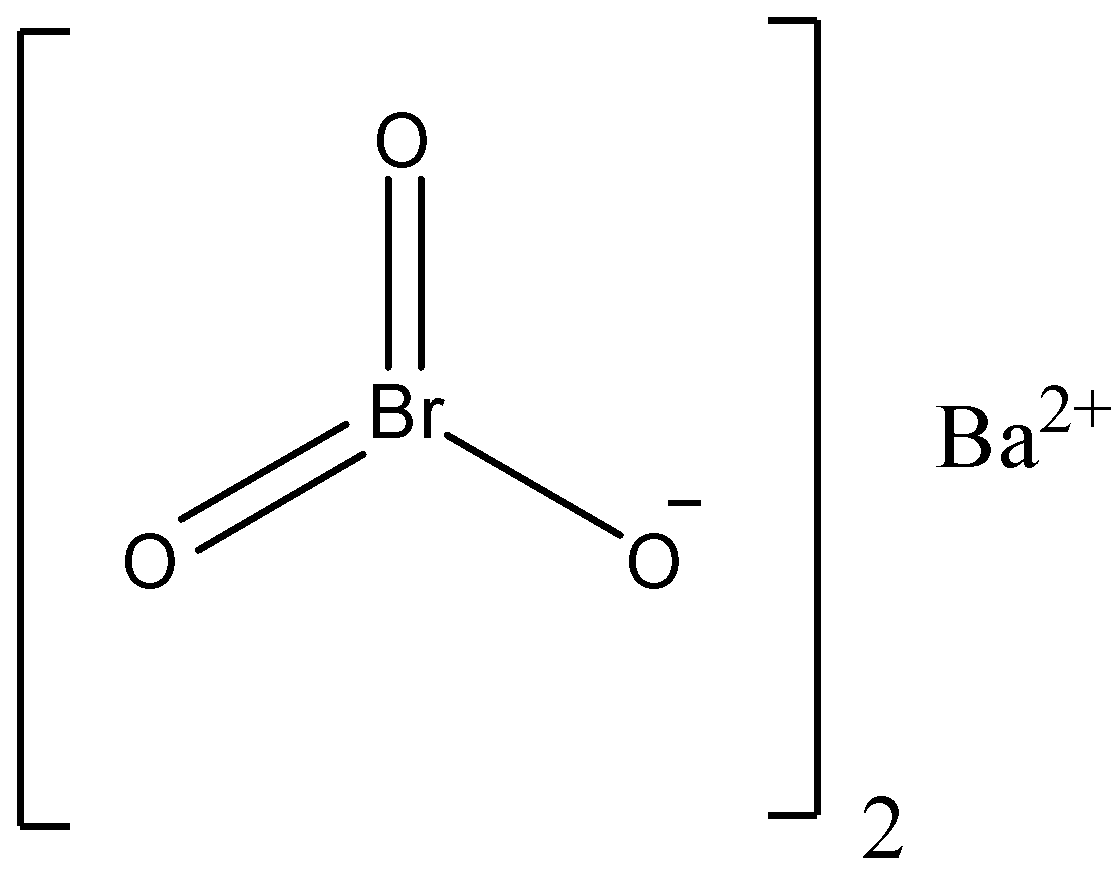
We must know that the barium bromate is mainly used as an oxidizing agent and analytical reagent and it is widely used to prepare other bromates like bromic acid, alkali metal bromates, etc. The barium bromate can be prepared by the reaction of hydroxide with sodium bromate and the reaction can be written as,
Ba(OH)2+2NaBrO3→Ba(BrO3)2+2NaOH
Note: Barium bromate is a white crystalline solid and the chemical form for barium bromate can be written as Ba(BrO3)2. We have to remember that the barium bromate is slightly soluble in water and is mainly used as a corrosion inhibitor.
