Question
Question: What is the chemical equation for the combustion of \({{C}_{3}}{{H}_{6}}\)?...
What is the chemical equation for the combustion of C3H6?
Solution
To solve this question we first need to know what is combustion. When a substance is reacted with oxygen gas, it releases energy in the form of heat and light. This reaction is called a combustion reaction.
Complete answer:
We know that C3H6 is the molecular formula for propene.
Propene is also called methyl ethylene or propylene.
It is unsaturated as it has 1 double bond and is the second simplest alkene after ethylene.
It has a petroleum-like faint odor and is a colorless gas.
The skeletal structure of propene is as follows
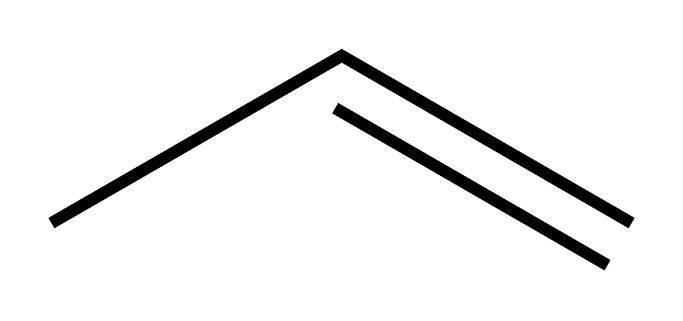
Propylene undergoes combustion reaction in a way similar to other alkenes.
- When there is sufficient oxygen, carbon dioxide and water is produced. The balanced chemical equation as follows
2C3H6+9O2→6CO2+6H2O
- When there is limited oxygen such that complete combustion cannot take place, water, carbon monoxide and soot in the form of carbon is produced. The balanced chemical equation is as follows
C3H6+2O2→3H2O+2C+CO
Note:
It should be noted that apart from propene, C3H6 also exists as cyclopropane.
In cyclopropane, there are 3 methylene CH2 groups attached to each other forming a ring. There exists a substantial ring strain due to the small size of the ring.
The skeletal structure of cyclopropane is as follows
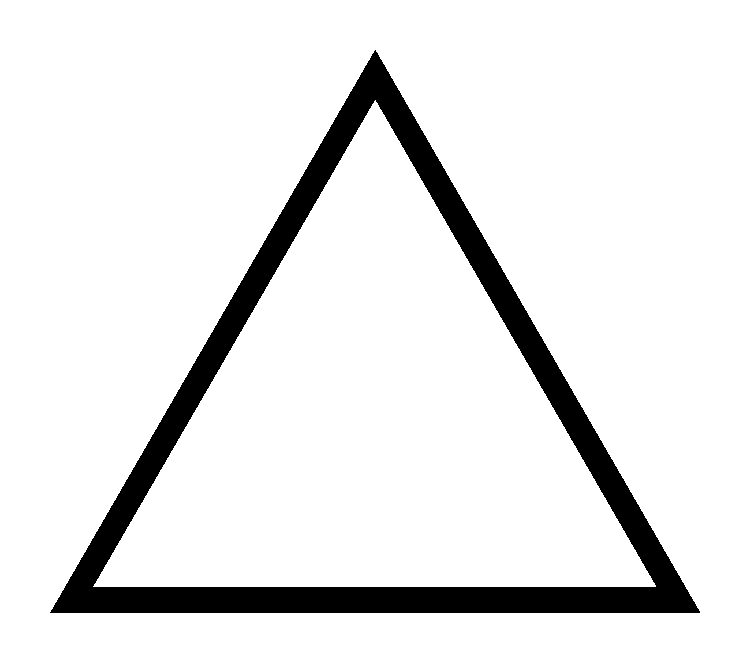
The products of combustion of cyclopropane are the same as the products formed during the combustion of propene i.e., water and carbon dioxide. The balanced chemical equation is as follows
2C3H6+9O2→6CO2+6H2O
