Question
Question: What is the change in oxidation number of Pb during conversion of $PbO_2$ to $Pb(NO_3)_2$ ?...
What is the change in oxidation number of Pb during conversion of PbO2 to Pb(NO3)2 ?
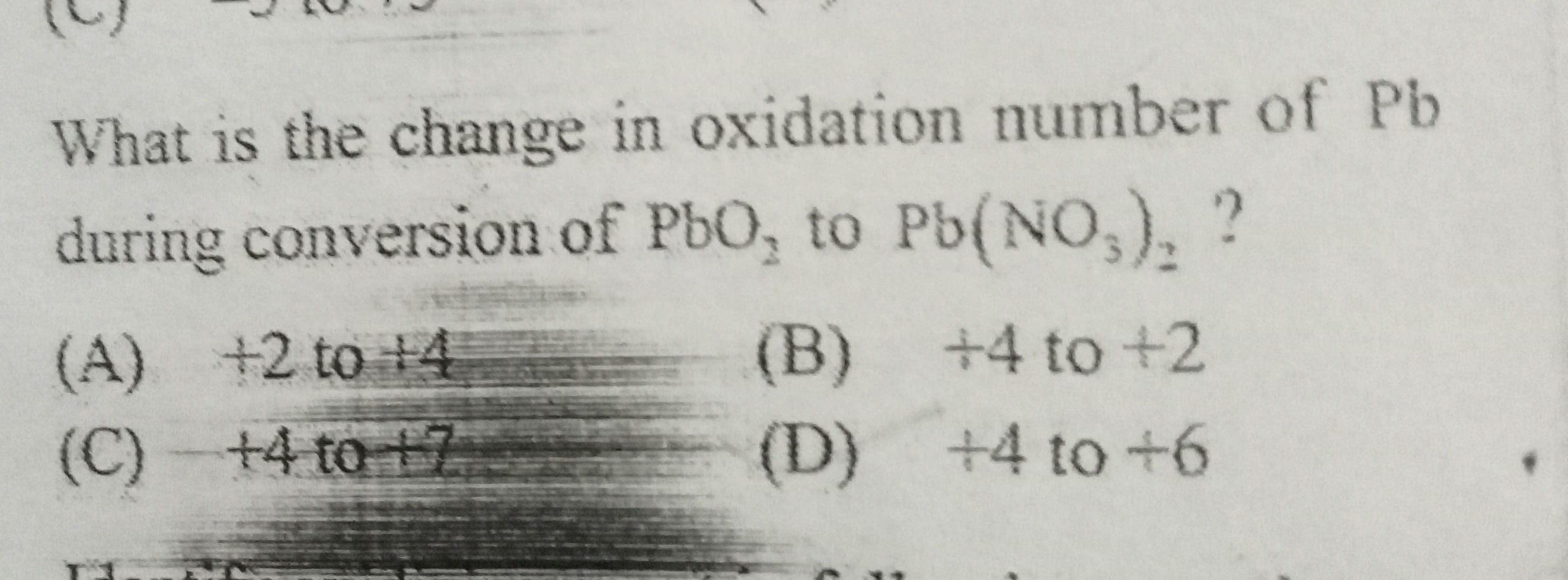
A
+2 to +4
B
+4 to +2
C
+4 to +7
D
+4 to +6
Answer
+4 to +2
Explanation
Solution
For PbO2:
Let oxidation state of Pb = x. With oxygen at −2,
x+2(−2)=0⟹x=+4.For Pb(NO3)2:
Since NO3− has a charge of −1, and there are 2 nitrate ions,
x+2(−1)=0⟹x=+2.Thus, the oxidation number of Pb changes from +4 to +2.
