Question
Question: What is the bond polarity of \[N{H_3}\]? A) \[N{H_3}\] is a non-polar molecule B) \[N{H_3}\] is ...
What is the bond polarity of NH3?
A) NH3 is a non-polar molecule
B) NH3 is a polar molecular
C) N−H bond is polar
D) Both B and C
Solution
We also have to know that the polarity comes when Hydrogen atoms are attached to an electronegative species. A molecule is said to be polar when the distribution of electrons between the atoms that are covalently bonded to it is not polar. On the other hand A nonpolar molecule is the one in which electrons are distributed equally.
Complete answer:
We will going to see the structure, hybridization and geometry before answering this question
We can draw the structure of ammonia as,
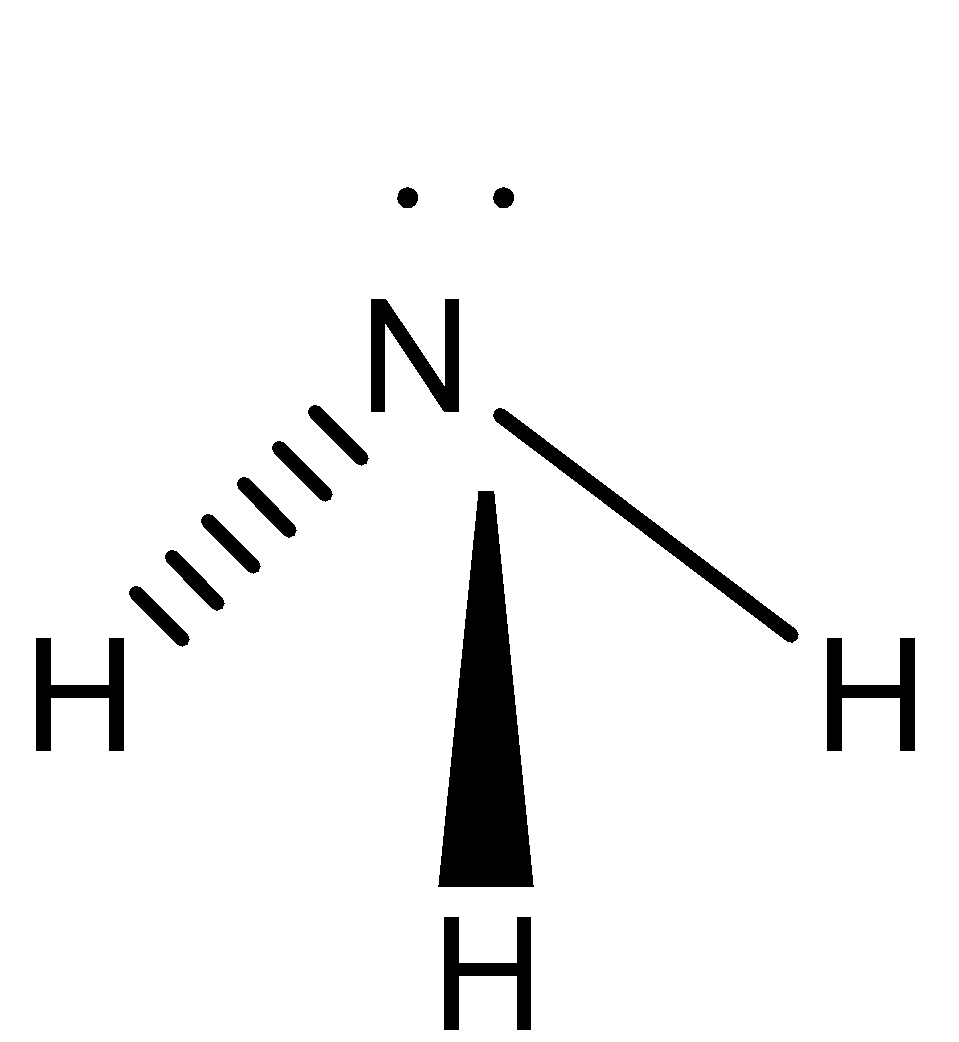
We also remember that the hybridization of ammonia is sp3
Molecular Geometry: Pyramidal
As we know that every N−H bond is polar in ammonia since the electronegativities of both Nitrogen and Hydrogen are different. The molecular geometry of ammonia is not symmetrical due to which it will not have 0 dipole moment and polarities will not be cancelled.
N will have partial negative charge while H atom will have partial positive charge. N is much more electronegative than the H atom, due to which ammonia turns out to be a polar molecule.
There are 3N−H bonds in ammonia and one lone pair of atoms on N.
The distance between N−H bond is 101.7pm and the angle between HNH is 107.8∘
Example of Polar molecule: NH3, H2O
Example of Non Polar molecule: CH3,NaCl
Option A) this is an incorrect option as ammonia is not non polar as discussed above.
Option B) this option is right as Ammonia is a polar molecule which we have discussed but we have other options too, so look at all the options
Option C) this option is right as N−H bond is polar due to the variant electronegativity between H and N, but we will look at other options too.
Option D) this is a correct option as it contains both the options which are right.
Note:
We have to remember that the bond polarity basically tells about the polarity of bond. Since ammonia is a polar molecule it will have polar bonds as well, which is N−H bond in this case.
